My Substack is a reader-supported publication. To gain access to all of my articles and podcast episodes in full and to allow me to continue devoting my time and effort to my writing, consider becoming a paid subscriber ($15 per month or $99 for a yearly subscription).
Disclaimer: Content for entertainment purposes only. Not medical or health advice.
“Low thyroid function is capable of disordering menses in many ways, beginning even with affecting its onset. Paradoxical as it may seem, it is a fact that hypothyroidism may either hasten the onset or delay it. It may bring on menstruation several years before the usual time. In one case reported in the medical literature, the girl was only five years and two months old when flow started and by age nine had fully developed breasts and pubic hair. At that point, a disturbance of thyroid function was suspected and thyroid therapy not only stopped the precocious menstruation but also led to regression of breast size and loss of pubic hair. Later, menstruation and development followed at the normal time.”
- From the book: “Hypothyroidism The Unsuspected Illness” by Broda O. Barnes, M.D. and Lawrence Galton
In 2022, The New York Times published a hit piece titled “Puberty Starts Earlier Than It Used To. No One Knows Why.”1
I am happy that this topic is getting mainstream traction and is no longer being ignored, as early puberty is far from a benign issue. Not only is it a serious issue at the level of the individual experiencing it, but it is also a serious issue at the societal level, as it’s indicating that there’s something extremely concerning happening as far as our general health as a species goes. This issue is also very close to my heart as I myself am a victim of early puberty, with my first menstrual period arriving at age 9.
However, the one piece that I strongly disagree with is the statement that “no one knows why” early puberty is becoming such a widespread issue.
The handful of articles that have popped up exploring this topic make the connection between abuse and early puberty, or specific environmental chemicals and early puberty, and note that kids who go through puberty earlier tend to suffer from mood disorders later in life. What these publications have failed to do so far is to draw the common thread that connects all of these factors. My goal with this article is to show that the effect of the various factors that trigger early puberty isn’t random as, biochemically, they all affect the body in a very similar way.
The “no one knows why” sentiment remains because no single environmental or socioeconomic factor is the entire cause of early puberty. Early puberty is the culmination of a “death by 1,000 cuts.”
Genius minds in health and physiology, such as Dr. Broda Otto Branes or Dr. Raymond Peat, as well as other researchers, have discovered the cause of early puberty decades ago. Low thyroid function, excessive exposure to estrogenic chemicals, and physiological and psychological stressors of any kind, not only compound onto one another but also potentiate the negative effects of each other, to hasten sexual maturity, while also accelerating the aging, degeneration and malfunction of every single system in the body.
This is because, at the biochemical level, early puberty is triggered by an excess of the adrenal hormones and estrogens,23 and all stressors (whether psychological or physiological, such as hypothyroidism) result in this hormonal pattern.
Early puberty is a bad omen as far as a person’s physical and mental development in the future goes, and the fact that it’s now a widespread phenomenon should have us very concerned. This is because early puberty is a sign of serious metabolic derangements and hormonal imbalances, which, if not corrected, will spiral into more serious issues over time.
In This Article:
Data showing that early puberty is becoming more common (and what constitutes an early puberty)
Why early puberty should concern us
How stress causes early puberty
The common thread between hypothyroidism, estrogenic overload and psychological stress
Abuse and a chaotic home are not prerequisites for early puberty
Compounding stressors make early puberty more likely
Early puberty could lead to improper development of secondary sexual characteristics
Estrogen dominance, progesterone deficiency, low thyroid function and tuberous breasts
Why early puberty is becoming more common
Childhood obesity is a risk factor for early puberty, but it’s not because we “eat more and move less”
Generational hypothyroidism has become more common since the invention of antibiotics
What to do to reverse this alarming trend
Limiting xenoestrogen and phytoestrogen exposure
Avoid dietary phytoestrogens and anti-thyroid compounds and cover your micronutrient needs
Addressing psychological stressors
Conclusion
Early Puberty Is Becoming More Common
In current literature, precocious (premature) puberty is defined as starting the development of secondary sexual characteristics in girls before age 8 and menstruating before age 10. In boys, puberty is considered precocious when it starts before age 9.
The average age at first menstruation in Western countries has steadily dropped from 16-17 in the late 1800s to 13 by 1980, and 11-12 today, seemingly continuing to drop.4 Similarly, the age of breast development in girls in the United States dropped from age 11 in the 1970s to 10 in 1990.5 A one-year difference in just 20 years is a massive change. By 2015, the average age at the onset of breast development in the United States dropped further to 9.5 years.6
This trend isn’t unique only to the West. Research showing similar trends is also coming out of other countries, such as Thailand,7 Mexico,8 and Turkey910, and the age of breast development has continuously dropped from 1975 to 2015 not only in North America but also in Europe, Asia, and the Middle East.


However, other than the average age of puberty dropping, precocious puberty is becoming more and more common.
Between 2008 and 2020, the rate of premature puberty in Korean children increased by 135.8% in boys and 118.5% in girls.11 The onset of early puberty has especially been accelerated during the COVID years (due to the stress of the pandemic and lockdown), with multiple studies from numerous countries (including Japan,12 China,13 Italy,14 Turkey and the United States) showing that precocious puberty is becoming alarmingly more and more common. For example, data from one clinic in San Diego, California shows that in 2020, the number of children brought to the clinic for the treatment of early puberty more than doubled in comparison to the previous 4 years.15 Research from Turkey also saw that the rates of early puberty in girls more than doubled during the lockdown.16
“In a society with high levels of child wellbeing, the threat to life presented by an unknown and potentially serious disease and the stressful environment created by lockdowns and other public health measures could trigger earlier pubertal maturation as an evolutionary response to favour early reproduction. The main driver for increased rates of precocious and rapidly progressive puberty during the pandemic could have been the environment of “fear and stress” in schools and households. In many children, CPP [central precocious puberty] may have been triggered by the psychological effects of living without normal social contact, using PPE, being near adults concerned about financial and other issues and the fear of getting ill.”17
Why Early Puberty Should Concern Us
On the psychosocial level, early puberty can come with a lot of baggage.
I can say from experience that going through puberty at an inappropriately young age has obvious social implications. For one, when you have the brain of a 9-year-old but the body of a 15 or 16-year-old, many unwanted advances from those who assume you to be older are bound to happen, all while lacking the needed social skills to truly grasp the situation at hand.
At the same time, going through puberty when all of your friends are still very much at the child stage of development is an extremely isolating experience, filled with shame, confusion, sudden feelings of otherness, and a general sense of lonely as none of your peers can relate to your current experience. When my period started, I was in the 4th grade, and I was the only child I knew to go through puberty at such an early age. This was in the early 2000s in my home country of Poland, a time and place when early puberty was not yet as common as it is now.
Not only does early puberty set a person up for a coming-of-age experience characterized by a fundamental disconnect from others your age as you are on two completely different timelines, but it also sets a person up for a lifetime of illness and chronic health problems, if the needed steps to course correct aren’t taken.
Apart from the psychosocial concerns around how experiencing sexual maturity too early in life can affect a child’s development, early puberty has been associated with an increased risk of numerous chronic, metabolic diseases, including diabetes, reproductive disorders like PCOS, cancers, autoimmune diseases (such as multiple sclerosis), heart disease, premature menopause, depression and anxiety.181920
This is because an excess of estrogen and cortisol are the driving forces behind both early puberty and all of the above-mentioned conditions.
How Stress Causes Early Puberty
“Stress-induced flowering is the ultimate adaptation to stress, because plants can survive as a species if they flower and produce seeds even if they cannot survive as an individual.”21
“When I worked in the woods, the foreman pointed out that you can make a pine tree produce cones the following season by thrashing it with an axe. It sensed danger and decided it had to reproduce. And puberty is similar. When the body senses that its metabolism is being slowed down, it turns on the reproductive system. And in extreme stress conditions, kids can start becoming pubescent well before the age of 10. Lots of girls in the US are starting to menstruate at nine, some are even earlier.” - Dr. Raymond Peat, 2019
In plants, flowering is the equivalent of puberty in humans. Flowering plants need to grow their flowers for reproduction.
When a plant is grown in a stressful environment, depending on the degree of stressors, it might adopt two different strategies. If the stress is too great, it may fail to flower entirely. However, if the stress is not as severe, it will enter the flowering stage prematurely. This is an adaptive strategy, in which the stressful stimuli from the environment signal to the plant that it might not be able to survive very long, so it should try and reproduce as soon as possible to ensure the survival of its species.
This is not at all different from what triggers early puberty in humans.
Early puberty is very much the body’s way of saying: “My environment is so stressful, volatile and unpredictable, that I don’t know if I’ll be able to survive much longer. Since my survival might be at risk, I should prioritize reproducing as soon as possible so that my lineage can continue.”
This grim reality is not just speculative, as it has been shown that, if course correction isn’t taken, early puberty can lead to a shorter reproductive life span and a shorter life expectancy.2223 This is not surprising, as the same biochemical factors that lead to early puberty are the exact same factors that lead to disease progression, eventual reproductive failure, and lastly death.
The Common Thread Between Hypothyroidism, Estrogenic Overload and Psychological Stress
At the level of the cell, the common thread between hypothyroidism, excess stress and estrogenic overload is that they all result in cells being unable to fully turn the foods we eat into energy, losing some of the food’s energy potential. As the body realizes that it lacks all the energy that it needs to maintain its structure, function and repair, alarm bells go off, pushing it towards prioritizing reproduction to ensure the continuity of its lineage.
Hypothyroidism, excess stress and estrogenic overload form feedback loops that feed into potentiating one another.
When the body experiences psychological stress, it ramps up the production of the adrenal hormones, such as cortisol, adrenaline and the androgen DHEA. These hormones are meant to help us survive acute “life or death” situations, by partitioning energy towards functions necessary for immediate survival and away from other functions.
When the stressor is chronic, such as when growing up in a home with socio-economic instability, abuse of any type, experiencing food or shelter scarcity, witnessing a heated divorce of parents/an abusive relationship among caregivers, or living in any other environment that’s otherwise volatile, the body remains in a state of chronic readiness for “fight or flight.” This chronic stress can cause the adrenal glands to enlarge and upregulate the production of the adrenal hormones,24 including the androgens which are involved in triggering early puberty in boys.
The rate at which our cells can produce energy out of the foods that we eat is controlled by the active thyroid hormone, T3, entering cells.
Cortisol inhibits the function of the enzyme that converts the thyroid prohormone T4 to the active thyroid hormone T3.25 In other words, high cortisol levels interfere with the creation of thyroid hormones. Adrenaline, another adrenal hormone released during fight or flight, can cause T4 to be converted to the inactive reverse T3 (rT3), which blocks the action of T3.26 Basically, chronic stress lowers thyroid function, leading to hypothyroidism.
Stressors also tend to elevate estrogen directly by upregulating the activity of an enzyme called estrogen synthase (aromatase).2728 This enzyme is expressed in most tissues, including the skin, bones and internal organs, but it’s most abundantly expressed in fat cells. It facilitates the conversion of the androgens that the adrenals produce during stress into estrogen.
In hypothyroidism, the clearance of cortisol is decreased and the body’s sensitivity to it is also decreased, which results in chronically elevated cortisol levels, even if a person isn’t stressed in the conventional sense of the word. Hypothyroidism has been shown to increase the activity of the estrogen-synthase (aromatase) enzyme.29
Since liver function is slowed in hypothyroidism, old estrogens (whether endogenous or environmental, such as the xenoestrogens from plastics) are removed from the body at a slower rate. The constipation caused by hypothyroidism can cause the estrogens that the liver metabolized and sent to the gut for excretion through the bowel to be reabsorbed and sent back into general circulation. In a feedback loop, estrogen acts on the adrenals to increase cortisol, lowering thyroid function further. In short, hypothyroidism leads to higher levels of estrogen and cortisol, and higher levels of estrogen and cortisol lead to hypothyroidism.
Since thyroid hormones control the rate at which fuel from food is converted into cellular energy, in a hypothyroid state, food is more likely to be deposited on the body as fat, as it fails to pass through all the stages of cellular respiration, where it would be partially converted into energy in the form of ATP and partially offloaded as heat. This means that in a hypothyroid state, a person has an easier time putting on fat. Since brain ATP controls hunger cues,30 a body that struggles to generate enough ATP due to hypothyroidism is more likely to over-eat, as satiety signalling becomes impaired. As mentioned earlier, the estrogen-synthase enzyme is mostly expressed in fat cells, meaning that if a person puts on excess fat due to hypothyroidism, they will create more estrogen.
Abuse and a Chaotic Home Are Not Prerequisites for Early Puberty
Experiencing abuse, volatility in the home, and similar stressors, often act as triggers for precocious puberty. However, not every child who experiences volatility in the home goes through early puberty, and not every child who goes through early puberty grows up in a chaotic home.
A child could go through early puberty because they are stressed in the conventional sense of the word (abuse, neglect, etc.), but they could also enter early puberty due to the increases in cortisol and estrogen caused by hypothyroidism, exposure to environmental estrogens, and excessive consumption of polyunsaturated fats, even if their home is peaceful and their caretakers fully attuned.
Exposure to environmental estrogens, from plastics, cosmetics, furniture offgassings, cleaning products, polyester clothing, and phytoestrogens from estrogenic plants, such as flaxseeds, soy, or the essential oils of tea tree or lavender, can equally contribute to the body’s estrogen load, increasing the synthesis of cortisol, lowering thyroid function, and triggering early puberty.31 Polyunsaturated fats, found in vegetable oils and conventional pork and poultry, similarly contribute to the issue, by interfering with the liver’s ability to remove estrogen32 and preventing thyroid hormones from entering cells.33
“Low thyroid function, relative over-feeding, and the presence of unsaturated oils in the diet are known to accelerate sexual maturity. Early sexual maturity has been associated with premature aging and early death.”
- from the book: “Generative Energy Restoring The Wholeness Of Life” by Dr. Ray Peat
Since stressors compound onto one another, if a child has a chaotic home while also being exposed to excess environmental estrogens, being fed a nutrient-poor diet high in polyunsaturated oils, and being exposed to chemicals that impair thyroid function (such as fluoride), they are at an extreme risk of early puberty.
The latter has been my case, and it explains perfectly why early puberty happened to me, in a time and place where it wasn’t yet so common.
In My Experience, Compounding Stressors Make Early Puberty More Likely
While I myself grew up in a home where shelter, food or resources were never a concern, my primary caretakers were often emotionally volatile and immature, with my home environment always feeling tense and never truly peaceful. My first period was eventually triggered when my mom and I became separated for a year due to her temporarily moving overseas; an event that I did not expect to affect me much as I was already living with my extended family, but which my subconscious mind and body unmistakenly registered as a major stressor.
Apart from the somewhat emotionally volatile home, I was born as at least the 3rd generation of hypothyroid women in my family.
My grandma, with whom I lived, made the switch to using exclusively vegetable oils in cooking decades before most people in her age group. For most of her adult life, she has been hypothyroid. My mom, having been brought up on this vegetable-oil-rich diet, suffered from estrogen dominance for a long time, failing to get pregnant for 10 years. The mother’s hormonal imprint and thyroid function affect her child in utero and tend to be inherited. This is actually part of the reason why mothers who experience pre-eclampsia (with pre-eclampsia being due predominantly to excessively high estrogen levels, progesterone deficiency, and pregnancy malnutrition) or gestational diabetes (which tend to be brought on by nutritional deficiencies and hypothyroidism) are more likely to give birth to children who end up going through puberty earlier.3435 Similarly, food scarcity in and before pregnancy (think famine, or, in modern times, a mom with a history of dieting excessively) can produce kids with lower metabolic rates, who are more prone to becoming obese.36
In my case, although I was born at term, I am without a doubt sure that I was already born mildly hypothyroid, having been imprinted on by my mother’s hormonal state in pregnancy.
Since early childhood, my body was already showing signs of hypothyroidism, such as rapid growth (I was a head and a shoulder taller than all the boys in my classes), water retention, a propensity to put on fat, endless respiratory infections, painful, cracking joints, sleep issues, phobias, and a general lack of energy and a tendency to shut down.
When a person is already hypothyroid and their biochemistry is already skewed towards estrogen dominance and energy conservation, they experience stressors more intensely than a person with a balanced biology. Their tolerance threshold is much lower.
I’m nearly sure that if I hadn’t already been born hypothyroid, I would probably not have found certain aspects of my family upbringing as stressful as I happened to find them. On the other hand, if I were to have been even more hypothyroid and estrogen dominant than I already was, even a peaceful, easy-going and joyful familial home likely wouldn’t have prevented an early onset puberty. However, when enough stressors compound onto one another, early puberty results. Sadly, so many aspects of our environments are stacked against us by now, that for many kids, it takes fewer and fewer stressors to trigger early puberty.
Early Puberty Could Lead To Improper Development of Secondary Sexual Characteristics
In women, the two hormones that need to be in intimate balance for puberty to progress as it should are estrogen (or rather the group of hormones called estrogens: estrone, estradiol and estriol) and progesterone. In men, the androgens (such as DHEA, testosterone, androsterone, and DHT) take the leading role.
The saying that “growth comes from adversity” is quite fitting for describing the function of estrogen. Estrogen levels elevate under stress and are involved in triggering puberty. Estrogen is also the main hormone responsible for growing fatty tissues, for example, stimulating the filling out of the breasts and hips in women during puberty.
While progesterone and the androgens are produced predominantly by the ovaries and testes in females and males respectively, estrogenic compounds from foods or chemical exposure can take on the role of estrogen in the body, kicking off puberty before the body has the resources to produce adequate levels of progesterone or the androgens to balance out this estrogen. This is especially likely to be the case when the person is hypothyroid, as the conversion of cholesterol into progesterone, pregnenolone and the androgens, and the release of progesterone by the ovary, are thyroid-hormone dependent. Additionally, estrogenic compounds themselves can interfere with the synthesis of these other hormones, both indirectly, by interfering with thyroid function, and directly, for example, by increasing the inactivation of androgens and interfering with the testes’ ability to make androgens.37
“Several of the things which cause early puberty and high estrogen, also tend to work against progesterone synthesis.” - Dr. Raymond Peat, PhD. Article: “Menopause and its causes”
Estrogen dominance at puberty can result in premature growth of breasts in women and breast hypertrophy. In men, it can result in gynecomastia. In both genders, it can result in excessive fat gain and even obesity.38
However, when the excess of estrogens becomes even larger, especially when progesterone, the androgens, and thyroid hormones are deficient, high estrogen levels at different stages of sexual maturation can result in deformities. Estrogen has a seemingly dose-dependent contradictory action, where an excess of it can kickstart sexual maturation and cause excessive growth, but an even bigger excess of it can retard growth and prevent the normal development of sexual characteristics.
Very high estrogen levels during puberty can arrest bone growth, resulting in a very short stature. They can also cause various bone deformities in both sexes.39
In boys, very high estrogen levels (in utero and during early puberty) can result in a failure of the testicles to mature. Sometimes, estrogen excess results in boys being born with the testicles inside their body and failing to exit the body cavity at puberty, a condition that often requires surgery.40 A high estrogen-to-androgen ratio and high xenoestrogen exposure during puberty can diminish penis growth.41
Estrogen Dominance, Progesterone Deficiency, Low Thyroid Function and Tuberous Breasts
In women, high estrogen levels coupled together with low thyroid hormone levels and low progesterone can result in a condition called tuberous breasts, where the mammary glands fail to develop, since both thyroid hormone and progesterone are needed for cell differentiation, to allow for the development of complex structures in the body.
In this condition, the breasts fail to grow in size (even with major weight fluctuations), don’t grow into a regular “breast shape,” breastfeeding later in life is often either impaired or not possible due to the lack of or improper development of the mammary glands, and the under-breast area curves up as opposed to curving down, as the cavity where breast fat would normally exist becomes fibrotic. Male-to-female transgender individuals undergoing estrogen-only hormone replacement therapy without any progesterone tend to grow tuberous breasts, giving rise to the term “trans breast.” The condition tends to resolve and the shape of the breast tends to normalize if they add in bio-identical progesterone (which rarely happens as many doctors still fail to recognize the importance of progesterone in developing and maintaining female sexual characteristics, instead putting MTF individuals on estrogen and androgen-blockers only, which can also lead to depression/mood disorders, blood clots, and many other complications).
Why Early Puberty Is Becoming More Common
The short answer is that our environment is becoming more and more toxic, and more and more estrogenic, while our ability to meet this toxic load with a nutrient surplus is diminishing.
Exposure to various endocrine-disrupting chemicals, such as phthalates, parabens, surfactants (such as PFAs and PFOAs, which contain fluoride and contribute to fluoride toxicity), organochlorines, certain pesticides, PCBs, PBDEs (fire retardants), plastics (including polyester), and heavy metals, can lead to early puberty.42 In a study done in Poland, it was found that girls exposed to higher levels of air pollution, including pollutants such as benzene and nitric oxide, started menstruating earlier.43
Many of these chemicals are considered obesogens, having the ability to cause a person to put on fat, even if eating an amount of food that is entirely appropriate for their height, age and activity levels.44 These chemicals have multiple modes of action, including preventing thyroid hormones from entering cells, causing negative changes to the microbiome, stimulating fat cells to divide, and affecting thyroid-related gene expression.45 Research as far back as the 1970s showed that low-dose exposure to these types of chemicals resulted in weight gain in experimental animals on normal diets. Many of these obesogenic chemicals have been shown to pass down from parent to offspring,46 causing the offspring to put on more weight even if its food habits are normal.
Virtually all of these compounds are also estrogenic, being able to mimic the effects of estrogen in the body when absorbed through the skin, through inhalation, or ingestion. Chemically, it doesn’t take much for a molecule to start resembling estrogen and taking on an estrogen-mimicking role in the body. Most chemicals that are hydrophobic and have either a phenolic or aromatic ring in their structure can substitute for estrogen in the body to a lesser or greater extent. Some substances that fit this criteria include BPA (and other plastics), benzene, the synthetic estrogen DES (known for causing miscarriages and sexual deformities and reproductive cancers in the offspring of mothers given it), and the notoriously harmful pesticide DDT. Many of these, apart from being estrogenic, are also anti-androgenic, meaning that they can lower testosterone and other androgens in boys.47
“Because most of environmental chemicals, called estrogen disruptors or xenoestrogens, are toxic and estrogen/antiandrogen active, they can disregulate hypothalamic-pituitary-gonadal axis potentially inducing reproductive disorders.”48
Many heavy metals, such as cadmium, nickel, mercury, and aluminum are classified as metalloestrogens,49 due to their ability to mimic the actions of physiological estrogens. Heavy metals are a big constituent of air pollution, but they are also inconspicuously hiding in certain foods, due to non-ethical food production practices. Mercury, due to its insane toxicity and ability to kill everything, is used as an antimicrobial and some crop growers still use mercury-containing pesticides to prevent their crops from being eaten by insects. For example, one 2009 study found that high fructose corn syrup can be a factor in mercury exposure, due to the mercury-containing insecticides sprayed on the corn from which it’s derived.50
Vegetable oils have also been found to be sources of metals,5152 partially due to the manufacturing and purification process and partially because the seeds from which they are made tend to accumulate metals from the soil and fertilizers, and somewhere between 500 to 1,000 seeds are needed to make just one tablespoon of oil. This is in addition to the fact that the polyunsaturated fats found in vegetable oils slow down the liver’s ability to process and detoxify estrogens, lower thyroid function and are obesogenic themselves.53 In animal models, mothers who consumed more polyunsaturated omega-6 fats during pregnancy gave birth to hyper-estrogenic offspring with a proclivity to aggression and alcoholism later in life.54 In the last 100 years, the consumption of polyunsaturated fats has absolutely skyrocketed.
“A diet high in (n-6) PUFA increases circulating estrogen levels (Adlercreutz 1991), including during pregnancy (Hilakivi-Clarke et al. 1996b and 1997b).”55
Most ultra-processed foods tend to fall into the category of heavily contaminated foods, including ready-made baby foods and formula.5657
Some plants tend to accumulate certain metals or minerals from the soil even when grown organically. For example, tea tends to accumulate fluoride from the soil58 and brown rice tends to accumulate arsenic in its outer hull. In the animal kingdom, large, top-of-the-food-chain fish, such as sharks, swordfish or tuna, tend to naturally accumulate more mercury due to their consumption of smaller fish.
Aluminum or mercury59 adjuvants are used in vaccines as preservatives and to trigger immune activation at the site of injection.
The hormone-disrupting compounds are mostly fat-soluble, and a partial explanation for the increase in fatness among humans (and wild animals that live near humans)60, is that the fat tissue is acting as a storage/quarantine site for these chemicals. At the same time, as mentioned earlier, these chemicals alter metabolic pathways in ways that favour the deposition of food as body fat, as opposed to burning it for energy.
Childhood Obesity Is a Risk Factor for Early Puberty, but It’s Not Because We “Eat More and Move Less”
Childhood obesity is considered a big risk factor for going through early puberty, which makes absolute sense since fat tissue is a big contributor to endogenous estrogen and cortisol production. However, there is a reason why the condescending advice to “eat less and move more,” which is bombarding kids and adults alike from every direction, isn’t working.
As mentioned earlier, hypothyroidism and estrogen dominance, which are caused in large by stressors such as nutritional deficiencies and environmental pollutants, can make it easy to put on body fat on very little food, while not being able to actually derive energy from that food. If an excess of body fat meant an excess of energy, then overweight kids would be more energetic than thin kids, yet the opposite is true. They tend to be lethargic, melancholic, and often disengaged; all signs of low energy.
Of course, enjoyable forms of physical activity are very important for people of all ages. However, healthy kids who are not suffering from hypothyroidism, naturally want to engage in active play and novelty-seeking activities. If a child is reclusive and lethargic, it’s extremely unlikely that they are lazy, and very likely that they are hypothyroid.
While most “health” publications recommend increased physical activity as a cure-all, forced physical activity is stressful, and a hypothyroid child (or adult) is at an increased sensitivity to that stress. I can attest to that, as being a hypothyroid child myself, every time I tried to engage in sports in the first 10 years of my life, I would immediately come down with a respiratory illness afterwards and my joints would become creaky and swollen. It is only after addressing my hypothyroidism that I can now benefit, as opposed to suffer, from increasing my physical activity, playing sports and lifting weights.
Similarly, eating more is not why we are getting fat. Looking at some data on the daily caloric intakes of adults dating as far back as the 1500s, humans ate on average 2,500 to 5,000 calories daily.
Nutritional data from 50-200 years ago shows that the average human ate anywhere from 2,500-5,000 calories a day.616263
While the common argument is that people moved more in the past because they worked physical jobs, not all of them did, yet they ate plenty of calories and weren’t obese. The caloric intake of the ruling class, as well as bankers, lawyers, dentists, managers or real estate agents (in other words, people who were sedentary) was not lower.

“In 1950 the British Medical Association recommended four different levels of energy values for physical activity: 2,500 (sedentary), 3,000 (moderate), 3,500 (active), and 4,250 (very active) kilocalories respectively. These were reduced in 1969 and again in 1979.”64
“The predominant medical explanation continues to be that obesity is the result of a simple imbalance between excessive calorie intake and insufficient energy expenditure—the energy balance or “calories in, calories out” model. However, recent studies have demonstrated that this simple paradigm cannot explain the increase in BMI seen in recent years.”65
I would argue that these numbers show that the average caloric intake hasn’t changed much in Western countries over the last couple of centuries. If anything, one might argue that many eat fewer calories now.
Of course, not everyone in the past was slim. However, despite the total calories eaten by the average human remaining relatively the same over the last few centuries, obesity was far rarer in the past than it is now. This was well before exercise obsession took over the world, back when lifting weights was only done by a handful of people on the fringes of society, and far before anyone knew what Orangetheory is. It’s not the number of calories but the quality of foods eaten and the quality of our environment that has changed dramatically.
“A recent clinical study that strongly supports the obesogen hypothesis found that people with the highest blood levels of perfluorinated chemicals had lower resting metabolic rates and regained weight faster after dieting than those with the lowest levels.”66
Obesity, and its complications, aren’t becoming an epidemic because we are “eating more and moving less.” They’re becoming an epidemic because we are being poisoned by the quality of our environment.
In the last 100 years, the degree to which our environment is being polluted with plastics and other xenoestrogens and obesogens has absolutely skyrocketed. The byproducts created by monocropping or the fast fashion industry are constantly polluting our soil, air, and water supply. Let’s not forget that these chemicals, if proper corrective steps aren’t taken, can accumulate in the body and pass from parent to offspring over generations.
All that being said, the solution isn’t to become so afraid of the world that one becomes a shut-in. As seen from the COVID years, the stress of turning everyone into shut-ins who feared the toxicity of the outside world did significant damage to the health of the general population, including the youngest generation. There are ways in which the body can be supported to face the modern world and become more resilient to it, which will be discussed in the next section.
Generational Hypothyroidism Has Become More Common Since the Invention of Antibiotics
As grim as this may sound, another part of the reason why such a massive portion of the population is hypothyroid nowadays, including children, is because due to the discovery and distribution of antibiotics, hypothyroid people are now making it into adulthood.
Hypothyroid individuals are at an increased risk of succumbing to and dying from infections.67 In contrast, when exposed to the same infective agent, individuals with good thyroid function will likely be immune to it. Before antibiotics were invented, hypothyroid individuals simply got…weeded out before they could reach reproductive age.
In adulthood, if hypothyroidism is still not addressed, hypothyroid individuals are more likely to marry and reproduce with other hypothyroid individuals, creating hypothyroid children. Think about it. If you’re a lethargic, low-energy human, who maybe can only muster up enough energy to perform well at your job, and only have enough energy after work to drop on the couch and watch TV, you probably won’t make a happy couple with a high energy individual who’s ready to play sports or go for a hike after a long work day. You will likely instead get together with another low-energy, hypothyroid individual who matches your lifestyle needs.
“This idea is not as strange as it may seem at first blush. If we consider the fact that unless a courtship is a whirlwind affair, individuals with similar amounts of energy tend to pair off. It would be difficult for a low thyroid person with a tendency to be more easily fatigued and to require more sleep to date a normal person several nights a week and still function adequately by day; and the normal partner, if attracted at first to the other, would be likely soon to have second thoughts. If, in fact, individuals with marked differences in energy endowment should marry, some would, sooner or later, seek divorce.
I recall one patient, a man who had been grossly overweight and had had just enough energy to get through a day's work. With thyroid therapy and change of diet, he lost weight, became energetic, and then returned almost a year later complaining that he couldn't go on with his wife. She was tired, cross, unwilling to go out evenings.
When he was reminded that he used to come home from work, eat, read a paper, and go to bed, he realized that the present incompatibility had grown out of the change in his energy level, and that perhaps what was needed was some attention to the thyroid status of his wife.” - From the book: “Hypothyroidism The Unsuspected Illness” by Broda O. Barnes, M.D. and Lawrence Galton
This isn’t to say that individuals like myself, who grew up hypothyroid and were extremely prone to infections, should have just succumbed to the plague to make the world a better place. Hypothyroidism isn’t encoded in our genes. It’s not something that some of us were selected to suffer from. It results from an accumulation of stressors across generations (including toxins, deficiencies and suboptimal hormonal imprints), where nothing had been done to course correct. Toxins and hormones pass from the placenta and breast milk to the baby, so if our parents did nothing to reduce toxin levels, replenish nutrients, and de-stress (stress increases the synthesis of estrogen in the placenta),68 their disease state will be passed onto us.
Even most diseases that we are taught to be truly “encoded in the genes” aren’t, and are instead the body’s response to its suboptimal environment. For example, research shows that discontinuing the use of phthalate and paraben-containing makeup and beauty products and switching to cleaner products can turn off “cancer genes” and promote the expression of “normal” genes instead.69
“Expression of 19/26 (73%) known cancer-associated genes within this profile display transcriptional shifts to a relatively ‘normal’ profile demonstrating that the REDUXE protocol reverses and thereby reduces high-risk phenotypes in healthy cells, promoted and maintained by the use of usual XE [xenoestrogen] level PCPs. [personal care products]”
Course correction is always possible. However, just as the causative factors in hypothyroidism and all its consequences (estrogen dominance, higher stress, early puberty, chronic illness later in life) are a situation of a “death by 1,000 cuts,” the solution is similarly a “fix by 1,000 steps.” There is no magic bullet that can be used, and the issue has to be addressed truly holistically.
What to Do to Reverse This Alarming Trend
Preventing or reversing early puberty can rarely be done by focusing on only one aspect of health, such as nutrition. The main pillars of focus would need to be: limiting exposure to endocrine-disrupting chemicals, addressing nutrition, addressing psychological and socioeconomic stressors (or, more likely, helping to shift the perspective on them), and potentially addressing thyroid hypo-function directly.
Limiting Xenoestrogen and Phytoestrogen Exposure
The first step in addressing xenoestrogen exposure would be to address the personal care product that both your family and your child use. Anything scented (containing fragrance), including body sprays and perfumes, but also scented candles or scented laundry detergents, is nearly guaranteed to be full of xenoestrogens. Switching to unscented personal care products, cleaning products, and unscented beeswax candles would be the first step.
While making a switch away from artificial fragrances, many people become drawn to essential oils as a substitute. However, despite their natural allure, essential oils are far from harmless as far as the ability to negatively affect our hormones goes.
For example, to make approximately 15 mL of lavender essential oil, up to 2 kilograms of lavender might be needed. Plants contain plant estrogens (phytoestrogens), some more than others. The process of turning multiple kilograms of a certain plant into an extremely condensed solution results in concentrating massive amounts of plant estrogens into a few drops of oil. Many people who tend to dismiss anything alternative as a placebo assume that essential oils “do nothing,” when in reality, essential oils are potent chemical substances that should not be used in careless ways, and, in my opinion, are best not applied to the skin, nor ingested, unless the same care is applied as when using pharmaceutical drugs and a specific therapeutic outcome is sought after. Research has linked the use of lavender and tea tree essential oils and products containing these oils to gynecomastia in boys and early breast development in girls.7071 These signs of precocious puberty/estrogen dominance reversed once the use of these oils was discontinued.
The use of plastics should be limited as much as possible, especially storing food in plastic containers and plastic bottles. Avoid heating foods in plastic containers. Silicone zip-loc bags and silicone cutting boards can act as a replacement, however, storing food in glass containers and using wooden cutting boards would be ideal.
Try to avoid polyester clothing and polyester fabrics in the home as much as possible. Go for cotton, linen or wool clothing, blankets, and carpets. Since this switch can be difficult to make, prioritize switching to cotton undergarments (ideally organic cotton) as the main priority.
If you have a room with plenty of artificial fabrics, air the room out often (if living in a place with good air quality) or consider using an air filter. Cleaning the home often can help, as microplastics from artificial fabrics can accumulate in dust.
Looking into water filtration devices (both for drinking and bathing water) is equally as important, especially in areas where water is fluorinated. Water quality standards differ significantly from country to country, region to region and even city to city. The concern of pesticides and industrial waste products contaminating drinking water has been long known. This is becoming compounded by the contamination of the water supply by drug residue (from birth control pills, HRT, hormones used in factory farming and the like)72. I believe that steering on the side of safety and filtering our water ourselves is a smart thing to do whether or not we believe the water in our district to be safe and adequately filtered. There is no shortage of stories of inadequate testing standards with civilians and authorities only becoming aware of how contaminated tap water in a certain area is once the damage has been done.
Fluoride has a direct anti-thyroid effect, interfering with the absorption of iodine, a vital component of thyroid hormones.73 Filtering water in areas where water is fluorinated, together with avoiding additional sources of fluoride exposure, such as fluoride-containing toothpaste, excessive use of non-stick pans and excessive tea drinking, should be emphasized.
Some Clean Personal Care & Home Care Products Include:
Soap, shampoo, skincare:
Deodorant:
The skin under our armpits is more absorbent than most other areas of the skin, so using a clean deodorant is paramount.
Cleaning products:
+ vinegar & baking soda go a long way
Toothpaste:
Water & shower filters:
Big Berkey or Travel Berkey (I use a Travel Berkey myself. Make sure to rinse the additional fluoride filters well before using and don’t over-screw them during the attachment process to prevent aluminum from leaching into water. Watch this video for reference).
Avoid Dietary Phytoestrogens and Anti-thyroid Compounds and Cover Your Micronutrient Needs
Foods particularly rich in phytoestrogens (plant estrogens), as well as polyunsaturated oils, are best entirely avoided. These foods include soy products, flax seeds, sesame and sesame oil, and all oils and fats that remain liquid after being placed in the fridge. Most nuts and seeds are high in omega-6 fats, meaning that they are best eaten sparingly/as a garnish, or avoided. Certain herbs tend to be quite estrogenic, such as hops, red clover and black cohosh. Wheat can have an estrogenic effect for some, so varying your starch sources (for example, with foods such as potatoes or yams in place of bread or pasta) to avoid having wheat daily can be helpful.
Replace liquid cooking oils with butter, ghee, and coconut oil. For cold meals, use an extra virgin olive oil that turns solid when placed in the fridge. Read labels on your foods to avoid polyunsaturated oils. Most foods canned in oil and most pre-made spreads or salad dressings are full of polyunsaturated oils.
Raw cruciferous vegetables (cabbage, kale, cauliflower, broccoli, turnips, rapeseed), maca root powder, lima beans, flaxseeds, sorghum, sweet potato, cassava, soy and millet contain compounds called goitrogens, which can interfere with thyroid function either by inhibiting iodine uptake or by inhibiting the enzyme thyroid peroxidase which is needed to make thyroid hormones. These compounds are reduced by thorough cooking, so if choosing to consume cruciferous vegetables, cook them thoroughly and don’t consume them raw or undercooked. If consuming the other foods above (such as sweet potatoes, cassava or millet) be sure to also cook them for a long time and vary them with other starch sources to avoid making them daily dietary staples.
Iodine, selenium, iron and vitamin A (retinol) are the key nutrients needed for the synthesis of thyroid hormones. However, all of these nutrients (especially iodine, iron and retinol) are best obtained from food, as supplementing them can be dangerous in certain scenarios.
Iodine has to be in perfect balance with selenium. Otherwise, iodine on its own can cause oxidative damage to the thyroid by increasing hydrogen peroxide levels. Glutathione, the body’s key antioxidant which prevents injury caused by hydrogen peroxide, runs on selenium. In places where the use of iodized salt is widespread but the right dietary measures to obtain selenium from food aren’t met, salt iodization is associated with increasing the rates of hypothyroidism and thyroid dysfunction, by causing oxidative stress to the thyroid. I discuss this in more detail in my article on Hashimoto’s disease.
Selenium and iodine are best obtained from seafood, such as cod, shrimp, clams, halibut, mussels, oysters, octopus, and crab. Extra iodine can be obtained from occasional seaweed consumption. Kidneys (beef, lamb, goat) are an excellent source of selenium. Desiccated kidney can be bought as a supplement. Eggs are another good selenium source.
If you decide to supplement iodine, I’d always pair it with a selenium supplement.
The enzyme thyroid peroxidase, which synthesizes thyroid hormones, is iron-dependent. However, iron is another nutrient where an excess of it can cause the same if not greater damage as a deficiency would. Since iron is a very reactive metal, an excess of it can contribute to high levels of oxidative stress, leading to accelerated aging and conditions such as diabetes or liver injury. Iron’s potential to do damage is much greater in a body full of polyunsaturated fats, as iron can facilitate the oxidation of these fats in the body into toxic lipid peroxides and cellular junk in the form of lipofuscin. This means that iron is best obtained from food, as opposed to from supplements, to prevent over-correction. Iron is best obtained from foods such as chicken liver or chicken liver pate, which also contain copper, vitamin A and the B vitamins needed for proper iron handling in the body.
Sometimes, iron can be stuck in the body due to inflammation or due to a deficiency of the B vitamins, selenium, magnesium, or other cofactors in iron recycling. This can mean that a body with an iron excess can potentially show signs of poor iron availability and anemia. Lactoferrin, a protein found in raw milk, raw cheese and raw colostrum, has been found in multiple studies74 to increase hemoglobin levels in patients with low hemoglobin by lowering inflammation, posing as a safer alternative to iron supplements for those with low hemoglobin.
Vitamin A is needed to stimulate the thyroid gland to release thyroid hormones. However, due to the unsaturated nature of vitamin A, it can compete for transport proteins against thyroid hormones, interfering with their uptake by cells. Additionally, when certain nutrients are lacking, the body might not be able to use vitamin A well, resulting in the accumulation of inactive vitamin A metabolites in the body.
The need for vitamin A mirrors the thyroid function of an individual. A healthy individual with very good thyroid function will have a high need for vitamin A and will rapidly burn through their vitamin A reserves. A hypothyroid individual’s need for vitamin A will be lower, and they will be especially bad at handling the plant form of vitamin A (beta-carotene), as apparent by the orange calluses that hypothyroid individuals tend to develop.
“The conversion of beta-carotene (provitamin A) to 2 molecules of vitamin A (retinol) is accelerated by thyroxine and hyperthyroidism, respectively. The characteristic yellow tint of the skin in hypothyroidism is due to hyper-beta-carotenemia.”75
In hypothyroid individuals, foods very high in beta-carotene, such as sweet potatoes, carrots or carrot juice, are best limited. High oxalate foods, such as spinach and almonds, can interfere with the body’s ability to handle vitamin A. The body also needs adequate vitamins B3, B6, B12 and magnesium to be able to handle vitamin A and prevent toxic breakdown products of vitamin A metabolism from building up in the body.76 Chicken liver and chicken liver pate are preferred vitamin A sources as they contain the performed form of vitamin A (retinol) as opposed to beta-carotene, together with vitamins B3, B6 and B12. Eggs and goat milk are other vitamin A (retinol) sources that also contain many of the co-factors needed for vitamin A metabolism.
For the body to get rid of estrogen and produce the other sex steroids (such as progesterone and the androgens), it needs:
The amino acids taurine, glycine, methionine, cysteine and glutamine, found in animal products, especially gelatinous meats, scallops and hearts.
B vitamins (especially B2 and B6), found in liver, eggs, shellfish, chicken, bee pollen and ripe tropical fruit (such as mangoes and cherimoyas, which are high in B6).
Magnesium, found in ripe fruit, goat milk, and oysters. Enough magnesium can be hard to obtain via diet alone, and hypothyroid individuals tend to lose a lot of magnesium, so a magnesium supplement can be helpful.
Zinc, found in red meat and oysters.
Vitamin E, found in extra virgin olive oil, kiwis, and bell peppers. Similarly, vitamin E can be hard to obtain via diet alone, so a supplement can be helpful.
Indole-3-carbinol, found in high amounts in broccoli sprouts (which are also less goitrogenic than other cruciferous vegetables).
Limonene, found in the peel of citrus fruits, in foods such as orange or lemon peel marmalade.
Multiple, daily bowel movements. Oats can be helpful in binding to and helping to excrete estrogens via the bowel.77 Constipation is caused by hypothyroidism. A magnesium supplement and/or emodin, found in rhubarb stalks/rhubarb marmalade, aloe vera inner leaf juice, and low-dose senna tea, can act as safe laxatives to support daily bowel movements until thyroid function improves.
Sweating, through saunas, activity, or hot baths.
In medical practice, drugs that inhibit the estrogen synthase (aromatase) enzyme are most commonly used to reverse early puberty.7879
Multiple foods can act as aromatase inhibitors, especially thoroughly cooked white button, portobello or crimini mushrooms, chamomile, parsley, guavas, honey, propolis and mangosteen.
Vitamins D, E, A, and K2 (found in aged cheese, such as gouda and camembert, and dark chicken and goose meat) also act as aromatase inhibitors.
Sometimes, especially when low thyroid function is generational, a little extra push might be needed to get the body “up to speed" and get its functions, such as liver function and digestion, to pick up. In Dr. Broda Barnes’ work, this push was a desiccated thyroid supplement. He used desiccated thyroid to reverse precocious puberty, as shown in the quote at the very start of this article.
Shellfish (such as mussels or clams) are eaten as a whole animal and will contain a tiny bit of thyroid hormones. A soup made from the necks of pasture-raised chickens or turkeys should also contain some thyroid tissue. These are best obtained from a friendly farmer, as commercially sold chickens are likely to be hypothyroid enough themselves to not have much of a functioning thyroid gland left.
Minerals, such as selenium, magnesium, calcium (from dairy), and zinc protect the body against the accumulation of heavy metals and promote their excretion.
Lastly I want to emphasize that nutrients that increase thyroid function and help the body metabolize estrogens and endocrine-disrupting chemicals protect us against environmental exposure to toxins. Our environment is how it is, and becoming extremely neurotic and scared of everything will do more harm than good. Keeping the metabolic rate up and creating a nutrient surplus will protect us against that which we can’t control.
Addressing Psychological Stressors
Going back to the start of this article, in a susceptible child, stressors (isolation, volatile caregivers, bullying, etc.) can easily bring on early puberty, when the child is not given the proper tools to deal with such stressors.
Stress is an inevitable part of life. However, the extent to which we experience an event or a circumstance as stressful has a lot to do with our perception of the stressor. If we view the situation as stressful, we are more likely to be negatively affected by it. If we lack an attuned community that can support us during a time of adversity and when we are not taught that it’s in our ability to overcome or reframe the stressors that come our way, those stressors are much more likely to affect us negatively.
I believe that becoming attuned to a child’s needs, earning permission to enter their inner world, becoming a calm and reliable presence in their life and having an empathetic, non-judgemental ear to their problems, can go a massively long way in attenuating their stress, even if their living situation remains the same.
In Conclusion…
Early puberty becoming a worldwide phenomenon should be a wake-up call to us all. Its consequences are not just psychosocial, as it is a reflection of something very concerning happening with our hormones and our health on the species level.
Unfortunately, there is a concerning trend currently happening, with some health authorities wanting to change the definition of normal based on what is “common,” suggesting that perhaps since puberty is now happening earlier, we should shift the normal expected age range for puberty onset down a few years. This is not an isolated phenomenon. We are becoming sicker as a species, and instead of noticing and addressing this pattern in a way that gets to the root cause (with the root cause being an environment that is becoming more poisoned each day), we are shifting ranges to “fit the new normal.”
In Dr. Broda Barnes’ work, it was clearly illustrated that a resting mid-day core body temperature below 37 degrees Celsius/98.6 Fahrenheit is indicative of hypothyroidism and results in multiple unpleasant symptoms and seemingly unrelated disease states (early puberty included). Yet, since hypothyroidism is now an unrecognized epidemic and our core body temperature as a species has fallen (with nearly no one having a resting core body temperature of 37 degrees anymore), health authorities are now suggesting that we should lower the range for what is considered a normal human core body temperature.80 We are devolving and normalizing it.
Of course, I doubt that this article will reach those who might need this information most, nor do I expect it to have much of a change on the general societal trend of pollution, soil depletion, and promotion of phytoestrogen-heavy and nutritionally poor plant-based diets centred around mono-crops and vegetable oils. However, hopefully, this article will give you some tools that you can use for your well-being, as at this point, it’s on every one of us to look out for ourselves and stop this alarming trend, at least for our own families.
My Substack is a reader-supported publication. To gain access to all of my articles and podcast episodes in full and to allow me to continue devoting my time and effort to my writing, consider becoming a paid subscriber ($15 per month or $99 for a yearly subscription).
Some of my other articles:
Disclaimer: This content is not intended as treatment or support for any medical condition. Content for entertainment purposes only. Not medical or health advice.
https://www.nytimes.com/2022/05/19/science/early-puberty-medical-reason.html
Qian F, Shi N, Zhou H. Estrogen can promote the expression of genes related to precocious puberty in GT1-7 mouse hypothalamic GnRH neuronal cell line via activating G protein-coupled estrogen receptor. Gen Physiol Biophys. 2020 Jan;39(1):27-36. doi: 10.4149/gpb_2019049. PMID: 32039822.
Roy JR, Chakraborty S, Chakraborty TR. Estrogen-like endocrine disrupting chemicals affecting puberty in humans--a review. Med Sci Monit. 2009 Jun;15(6):RA137-45. PMID: 19478717.
Herman-Giddens, M.E. (2007). Puberty Is Starting Earlier in the 21st Century. In: Pescovitz, O.H., Walvoord, E.C. (eds) When Puberty is Precocious. Contemporary Endocrinology. Humana Press. https://doi.org/10.1007/978-1-59745-499-5_5
Marcia E. Herman-Giddens, Eric J. Slora, Richard C. Wasserman, Carlos J. Bourdony, Manju V. Bhapkar, Gary G. Koch, Cynthia M. Hasemeier; Secondary Sexual Characteristics and Menses in Young Girls Seen in Office Practice: A Study from the Pediatric Research in Office Settings Network. Pediatrics April 1997; 99 (4): 505–512. 10.1542/peds.99.4.505
Eckert-Lind C, Busch AS, Petersen JH, Biro FM, Butler G, Bräuner EV, Juul A. Worldwide Secular Trends in Age at Pubertal Onset Assessed by Breast Development Among Girls: A Systematic Review and Meta-analysis. JAMA Pediatr. 2020 Apr 1;174(4):e195881. doi: 10.1001/jamapediatrics.2019.5881. Epub 2020 Apr 6. PMID: 32040143; PMCID: PMC7042934.
Jaruratanasirikul S, Chanpong A, Tassanakijpanich N, Sriplung H. Declining age of puberty of school girls in southern Thailand. World J Pediatr. 2014 Aug;10(3):256-61. doi: 10.1007/s12519-014-0472-2. Epub 2014 Mar 25. PMID: 24668235.
Marván ML, Catillo-López RL, Alcalá-Herrera V, Callejo DD. The Decreasing Age at Menarche in Mexico. J Pediatr Adolesc Gynecol. 2016 Oct;29(5):454-457. doi: 10.1016/j.jpag.2016.02.006. Epub 2016 Feb 23. PMID: 26915923.
Ulubay M, Fidan U, Ozturk M. The decreasing age of menarche in Turkey: global warming, socioeconomic development, and environmental factors. Eur Rev Med Pharmacol Sci. 2023 Jul;27(14):6780-6784. doi: 10.26355/eurrev_202307_33148. PMID: 37522688.
Güran T, Helvacıoğlu D, Gürpınar Tosun B, Yavaş Abalı Z, Alır F, Arslan YT, Molla G, Şahin B, Sayar ME, Atay Z, Haliloğlu B, Demir K, Turan S, Hıdıroğlu S, Bereket A. Decline in the Age of Menarche in Istanbul Schoolgirls Over the Last 12 Years. J Clin Res Pediatr Endocrinol. 2023 May 29;15(2):154-159. doi: 10.4274/jcrpe.galenos.2023.2022-11-16. Epub 2023 Jan 26. PMID: 36700465; PMCID: PMC10234061.
Article Source: Ongoing increasing trends in central precocious puberty incidence among Korean boys and girls from 2008 to 2020, Kang S, Park MJ, Kim JM, Yuk JS, Kim SH (2023) Ongoing increasing trends in central precocious puberty incidence among Korean boys and girls from 2008 to 2020. PLOS ONE 18(3): e0283510. https://doi.org/10.1371/journal.pone.0283510
Matsubara K, Higuchi S, Watanabe Y, Kitayama K, Yamada Y, Yorifuji T. Increased frequency of central precocious puberty during the coronavirus disease (COVID-19) pandemic at a single center in the Osaka Metropolitan Area of Japan. Clin Pediatr Endocrinol. 2023;32(1):58-64. doi: 10.1297/cpe.2022-0008. Epub 2022 Dec 20. PMID: 36761494; PMCID: PMC9887298.
Chen Y, Chen J, Tang Y, Zhang Q, Wang Y, Li Q, Li X, Weng Z, Huang J, Wang X, Liu S. Difference of Precocious Puberty Between Before and During the COVID-19 Pandemic: A Cross-Sectional Study Among Shanghai School-Aged Girls. Front Endocrinol (Lausanne). 2022 Mar 21;13:839895. doi: 10.3389/fendo.2022.839895. PMID: 35392135; PMCID: PMC8979840.
Stagi S, De Masi S, Bencini E, Losi S, Paci S, Parpagnoli M, Ricci F, Ciofi D, Azzari C. Increased incidence of precocious and accelerated puberty in females during and after the Italian lockdown for the coronavirus 2019 (COVID-19) pandemic. Ital J Pediatr. 2020 Nov 4;46(1):165. doi: 10.1186/s13052-020-00931-3. PMID: 33148304; PMCID: PMC7609833.
Trujillo MV, Rungvivatjarus T, Klein KO. Incidence of central precocious puberty more than doubled during COVID-19 pandemic: Single-center retrospective review in the United States. Front Pediatr. 2022 Nov 30;10:1007730. doi: 10.3389/fped.2022.1007730. PMID: 36533230; PMCID: PMC9748187.
Acar, Sezer and Özkan, Behzat. "Increased frequency of idiopathic central precocious puberty in girls during the COVID-19 pandemic: preliminary results of a tertiary center study" Journal of Pediatric Endocrinology and Metabolism, vol. 35, no. 2, 2022, pp. 249-251. https://doi.org/10.1515/jpem-2021-0565
Street ME, Ponzi D, Renati R, Petraroli M, D'Alvano T, Lattanzi C, Ferrari V, Rollo D, Stagi S. Precocious puberty under stressful conditions: new understanding and insights from the lessons learnt from international adoptions and the COVID-19 pandemic. Front Endocrinol (Lausanne). 2023 May 2;14:1149417. doi: 10.3389/fendo.2023.1149417. PMID: 37201098; PMCID: PMC10187034.
Lauridsen LLB, Arendt LH, Ernst A, Brix N, Parner ET, Olsen J, Ramlau-Hansen CH. Maternal diabetes mellitus and timing of pubertal development in daughters and sons: a nationwide cohort study. Fertil Steril. 2018 Jul 1;110(1):35-44. doi: 10.1016/j.fertnstert.2018.03.014. Epub 2018 Jun 13. PMID: 29908773.
Azimi A, Hanaei S, Sahraian MA, Mohammadifar M, Ramagopalan SV, Ghajarzadeh M. Age at menarche and risk of multiple sclerosis (MS): a systematic review and meta-analysis. BMC Neurol. 2019 Nov 14;19(1):286. doi: 10.1186/s12883-019-1473-5. PMID: 31727014; PMCID: PMC6854684.
Goldberg M, D'Aloisio AA, O'Brien KM, Zhao S, Sandler DP. Pubertal timing and breast cancer risk in the Sister Study cohort. Breast Cancer Res. 2020 Oct 27;22(1):112. doi: 10.1186/s13058-020-01326-2. PMID: 33109223; PMCID: PMC7590599.
Camille Salmon, Sandrine Isnard, Yves Caraglio, Patrick Heuret, Architectural traits underlie growth form diversity and polycarpic versus monocarpic life histories in Cerberiopsis (Apocynaceae), Botanical Journal of the Linnean Society, Volume 202, Issue 4, August 2023, Pages 510–528, https://doi.org/10.1093/botlinnean/boad007
Mishra GD, Pandeya N, Dobson AJ, Chung HF, Anderson D, Kuh D, Sandin S, Giles GG, Bruinsma F, Hayashi K, Lee JS, Mizunuma H, Cade JE, Burley V, Greenwood DC, Goodman A, Simonsen MK, Adami HO, Demakakos P, Weiderpass E. Early menarche, nulliparity and the risk for premature and early natural menopause. Hum Reprod. 2017 Mar 1;32(3):679-686. doi: 10.1093/humrep/dew350. PMID: 28119483; PMCID: PMC5850221.
Xing Z, Alman AC, Kirby RS. Premature Menopause and All-Cause Mortality and Life Span Among Women Older Than 40 Years in the NHANES I Epidemiologic Follow-Up Study: Propensity Score Matching Analysis. J Womens Health (Larchmt). 2023 Sep;32(9):950-959. doi: 10.1089/jwh.2023.0189. Epub 2023 Jul 12. PMID: 37439866.
Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 2006 Nov;291(5):E965-73. doi: 10.1152/ajpendo.00070.2006. Epub 2006 Jun 13. PMID: 16772325.
Heyma P, Larkins RG. Glucocorticoids decrease in conversion of thyroxine into 3, 5, 3'-tri-iodothyronine by isolated rat renal tubules. Clin Sci (Lond). 1982 Feb;62(2):215-20. doi: 10.1042/cs0620215. PMID: 7053919.
Nauman A, Kamiński T, Herbaczyńska-Cedro K. In vivo and in vitro effects of adrenaline on conversion of thyroxine to triiodothyronine and to reverse-triiodothyronine in dog liver and heart. Eur J Clin Invest. 1980 Jun;10(3):189-92. doi: 10.1111/j.1365-2362.1980.tb00019.x. PMID: 6783414.
Shors TJ, Pickett J, Wood G, Paczynski M. Acute stress persistently enhances estrogen levels in the female rat. Stress. 1999 Dec;3(2):163-71. doi: 10.3109/10253899909001120. PMID: 10938577.
Wangsheng Wang, Jianneng Li, Yuchun Ge, Wenjiao Li, Qun Shu, Haiyan Guan, Kaiping Yang, Leslie Myatt, Kang Sun, Cortisol Induces Aromatase Expression in Human Placental Syncytiotrophoblasts Through the cAMP/Sp1 Pathway, Endocrinology, Volume 153, Issue 4, 1 April 2012, Pages 2012–2022, https://doi.org/10.1210/en.2011-1626
Panno ML, Beraldi E, Pezzi V, Salerno M, De Luca G, Lanzino M, Le Pera M, Sisci D, Prati M, Palmero S, Bolla E, Fugassa E, Andò S. Influence of thyroid hormone on androgen metabolism in peripuberal rat Sertoli cells. J Endocrinol. 1994 Mar;140(3):349-55. doi: 10.1677/joe.0.1400349. PMID: 8182361.
MacLean DB, Luo LG. Increased ATP content/production in the hypothalamus may be a signal for energy-sensing of satiety: studies of the anorectic mechanism of a plant steroidal glycoside. Brain Res. 2004 Sep 10;1020(1-2):1-11. doi: 10.1016/j.brainres.2004.04.041. PMID: 15312781.
Lee JE, Jung HW, Lee YJ, Lee YA. Early-life exposure to endocrine-disrupting chemicals and pubertal development in girls. Ann Pediatr Endocrinol Metab. 2019 Jun;24(2):78-91. doi: 10.6065/apem.2019.24.2.78. Epub 2019 Jun 30. PMID: 31261471; PMCID: PMC6603611.
Lv X, Xia Y, Finel M, Wu J, Ge G, Yang L. Recent progress and challenges in screening and characterization of UGT1A1 inhibitors. Acta Pharm Sin B. 2019 Mar;9(2):258-278. doi: 10.1016/j.apsb.2018.09.005. Epub 2018 Sep 14. PMID: 30972276; PMCID: PMC6437557.
Benvenga S., Calzi L. L. and Robbins J.: Effect of FFA and nonlipid inhibitors of thyroid hormone binding in the immunoradiometric assay of thyroxine-binding globulin. Cl/n. Chem. 33 (1987) 1752-1755.
Persson I, Ahlsson F, Ewald U, Tuvemo T, Qingyuan M, von Rosen D, Proos L. Influence of perinatal factors on the onset of puberty in boys and girls: implications for interpretation of link with risk of long term diseases. Am J Epidemiol. 1999 Oct 1;150(7):747-55. doi: 10.1093/oxfordjournals.aje.a010077. PMID: 10512428.
Lauridsen LLB, Arendt LH, Ernst A, Brix N, Parner ET, Olsen J, Ramlau-Hansen CH. Maternal diabetes mellitus and timing of pubertal development in daughters and sons: a nationwide cohort study. Fertil Steril. 2018 Jul 1;110(1):35-44. doi: 10.1016/j.fertnstert.2018.03.014. Epub 2018 Jun 13. PMID: 29908773.
Parlee SD, MacDougald OA. Maternal nutrition and risk of obesity in offspring: the Trojan horse of developmental plasticity. Biochim Biophys Acta. 2014 Mar;1842(3):495-506. doi: 10.1016/j.bbadis.2013.07.007. Epub 2013 Jul 16. PMID: 23871838; PMCID: PMC3855628.
Tom O Abney, The potential roles of estrogens in regulating Leydig cell development and function: A review, Steroids, Volume 64, Issue 9, 1999, Pages 610-617, ISSN 0039-128X, https://doi.org/10.1016/S0039-128X(99)00041-0.
Laura C. Alonso; Robert L. Rosenfield (2002). Oestrogens and puberty. , 16(1), 13–30. doi:10.1053/beem.2002.0177
Laura C. Alonso; Robert L. Rosenfield (2002). Oestrogens and puberty. , 16(1), 13–30. doi:10.1053/beem.2002.0177
Rodprasert W, Virtanen HE, Mäkelä JA, Toppari J. Hypogonadism and Cryptorchidism. Front Endocrinol (Lausanne). 2020 Jan 15;10:906. doi: 10.3389/fendo.2019.00906. PMID: 32010061; PMCID: PMC6974459.
Mancini M, Pecori Giraldi F, Andreassi A, Mantellassi G, Salvioni M, Berra CC, Manfrini R, Banderali G, Folli F. Obesity Is Strongly Associated With Low Testosterone and Reduced Penis Growth During Development. J Clin Endocrinol Metab. 2021 Oct 21;106(11):3151-3159. doi: 10.1210/clinem/dgab535. PMID: 34283215.
Anastasiadis X, Matsas A, Panoskaltsis T, Bakas P, Papadimitriou DT, Christopoulos P. Impact of Chemicals on the Age of Menarche: A Literature Review. Children (Basel). 2023 Jul 17;10(7):1234. doi: 10.3390/children10071234. PMID: 37508731; PMCID: PMC10378553.
Wronka I, Kliś K. Effect of air pollution on age at menarche in polish females, born 1993-1998. Sci Rep. 2022 Mar 21;12(1):4820. doi: 10.1038/s41598-022-08577-3. PMID: 35315430; PMCID: PMC8938500.
Holtcamp W. Obesogens: an environmental link to obesity. Environ Health Perspect. 2012 Feb;120(2):a62-8. doi: 10.1289/ehp.120-a62. PMID: 22296745; PMCID: PMC3279464.
Egusquiza RJ, Blumberg B. Environmental Obesogens and Their Impact on Susceptibility to Obesity: New Mechanisms and Chemicals. Endocrinology. 2020 Mar 1;161(3):bqaa024. doi: 10.1210/endocr/bqaa024. PMID: 32067051; PMCID: PMC7060764.
Mohajer N, Du CY, Checkcinco C, Blumberg B. Obesogens: How They Are Identified and Molecular Mechanisms Underlying Their Action. Front Endocrinol (Lausanne). 2021 Nov 25;12:780888. doi: 10.3389/fendo.2021.780888. PMID: 34899613; PMCID: PMC8655100.
De Falco Maria, Forte Maurizio, Laforgia Vincenza, Estrogenic and anti-androgenic endocrine disrupting chemicals and their impact on the male reproductive system , Frontiers in Environmental Science, Volume 3, 2015, DOI:10.3389/fenvs.2015.00003, https://www.frontiersin.org/articles/10.3389/fenvs.2015.00003
Massart F, Parrino R, Seppia P, Federico G, Saggese G. How do environmental estrogen disruptors induce precocious puberty? Minerva Pediatr. 2006 Jun;58(3):247-54. PMID: 16832329.
Darbre PD. Metalloestrogens: an emerging class of inorganic xenoestrogens with potential to add to the oestrogenic burden of the human breast. J Appl Toxicol. 2006 May-Jun;26(3):191-7. doi: 10.1002/jat.1135. PMID: 16489580.
Dufault R, LeBlanc B, Schnoll R, Cornett C, Schweitzer L, Wallinga D, Hightower J, Patrick L, Lukiw WJ. Mercury from chlor-alkali plants: measured concentrations in food product sugar. Environ Health. 2009 Jan 26;8:2. doi: 10.1186/1476-069X-8-2. PMID: 19171026; PMCID: PMC2637263.
Yao, Li; Liu, Haitao; Wang, Xie; Xu, Wenzhi; Zhu, Yongqun; Wang, Hong; Pang, Liangyu; Lin, Chaowen (2018). Ultrasound-assisted surfactant-enhanced emulsification microextraction using a magnetic ionic liquid coupled with micro-solid phase extraction for the determination of cadmium and lead in edible vegetable oils. Food Chemistry, (), S0308814618303807–. doi:10.1016/j.foodchem.2018.02.132
Mehri, F., Heshmati, A., Ghane, E.T. et al. A probabilistic health risk assessment of potentially toxic elements in edible vegetable oils consumed in Hamadan, Iran. BMC Public Health 24, 218 (2024). https://doi.org/10.1186/s12889-023-17624-1
Simopoulos AP. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients. 2016 Mar 2;8(3):128. doi: 10.3390/nu8030128. PMID: 26950145; PMCID: PMC4808858.
Cabanes A, de Assis S, Gustafsson JA, Hilakivi-Clarke L. Maternal high n-6 polyunsaturated fatty acid intake during pregnancy increases voluntary alcohol intake and hypothalamic estrogen receptor alpha and beta levels among female offspring. Dev Neurosci. 2000 Sep-Dec;22(5-6):488-93. doi: 10.1159/000017480. PMID: 11111167.
Margarita Raygada, Elizabeth Cho, Leena Hilakivi-Clarke, "High Maternal Intake of Polyunsaturated Fatty Acids During Pregnancy in Mice Alters Offsprings’ Aggressive Behavior, Immobility in the Swim Test, Locomotor Activity and Brain Protein Kinase C Activity23,” The Journal of Nutrition, Volume 128, Issue 12, 1998, Pages 2505-2511, ISSN 0022-3166, https://doi.org/10.1093/jn/128.12.2505.
https://www.momsacrossamerica.com/baby_formula_toxic_metals_results_released
Bair EC. A Narrative Review of Toxic Heavy Metal Content of Infant and Toddler Foods and Evaluation of United States Policy. Front Nutr. 2022 Jun 27;9:919913. doi: 10.3389/fnut.2022.919913. PMID: 35832055; PMCID: PMC9271943.
Waugh DT, Potter W, Limeback H, Godfrey M. Risk Assessment of Fluoride Intake from Tea in the Republic of Ireland and its Implications for Public Health and Water Fluoridation. Int J Environ Res Public Health. 2016 Feb 26;13(3):259. doi: 10.3390/ijerph13030259. PMID: 26927146; PMCID: PMC4808922.
Kern JK, Geier DA, Homme KG, Geier MR. Examining the evidence that ethylmercury crosses the blood-brain barrier. Environ Toxicol Pharmacol. 2020 Feb;74:103312. doi: 10.1016/j.etap.2019.103312. Epub 2019 Dec 9. PMID: 31841767.
Klimentidis Yann C., Beasley T. Mark, Lin Hui-Yi, Murati Giulianna, Glass Gregory E., Guyton Marcus, Newton Wendy, Jorgensen Matthew, Heymsfield Steven B., Kemnitz Joseph, Fairbanks Lynn and Allison David B. 2011, Canaries in the coal mine: a cross-species analysis of the plurality of obesity epidemics, Proc. R. Soc. B.2781626–1632, http://doi.org/10.1098/rspb.2010.1890
https://fireinabottle.net/torpor-sloth-and-gluttony-part-1-americans-ate-a-lot-in-1939/
Segers, Yves. Economic Growth and Living Standards. Private Consumer Expenditure and Food Consumption in Nineteenth Century Belgium.
GIL, J. M.; GRACIA, A.; PEREZ, L. P. Y. (1995). Food consumption and economic development in the European Union. European Review of Agricultural Economics, 22(3), 385–399. doi:10.1093/erae/22.3.385
Leslie A. Clarkson, E. Margaret Crawford, “Feast and Famine: A History of Food in Ireland 1500-1920,” Oxford University Press, Year: 2001
Egusquiza RJ, Blumberg B. Environmental Obesogens and Their Impact on Susceptibility to Obesity: New Mechanisms and Chemicals. Endocrinology. 2020 Mar 1;161(3):bqaa024. doi: 10.1210/endocr/bqaa024. PMID: 32067051; PMCID: PMC7060764.
Egusquiza RJ, Blumberg B. Environmental Obesogens and Their Impact on Susceptibility to Obesity: New Mechanisms and Chemicals. Endocrinology. 2020 Mar 1;161(3):bqaa024. doi: 10.1210/endocr/bqaa024. PMID: 32067051; PMCID: PMC7060764.
“Hypothyroidism The Unsuspected Illness by Broda O. Barnes, M.D. and Lawrence Galton
Wangsheng Wang, Jianneng Li, Yuchun Ge, Wenjiao Li, Qun Shu, Haiyan Guan, Kaiping Yang, Leslie Myatt, Kang Sun, Cortisol Induces Aromatase Expression in Human Placental Syncytiotrophoblasts Through the cAMP/Sp1 Pathway, Endocrinology, Volume 153, Issue 4, 1 April 2012, Pages 2012–2022, https://doi.org/10.1210/en.2011-1626
Dairkee SH, Moore DH, Luciani MG, Anderle N, Gerona R, Ky K, Torres SM, Marshall PV, Goodson Iii WH. Reduction of daily-use parabens and phthalates reverses accumulation of cancer-associated phenotypes within disease-free breast tissue of study subjects. Chemosphere. 2023 May;322:138014. doi: 10.1016/j.chemosphere.2023.138014. Epub 2023 Feb 4. PMID: 36746253.
Henley DV, Lipson N, Korach KS, Bloch CA. Prepubertal gynecomastia linked to lavender and tea tree oils. N Engl J Med. 2007 Feb 1;356(5):479-85. doi: 10.1056/NEJMoa064725. PMID: 17267908.
Ramsey JT, Li Y, Arao Y, Naidu A, Coons LA, Diaz A, Korach KS. Lavender Products Associated With Premature Thelarche and Prepubertal Gynecomastia: Case Reports and Endocrine-Disrupting Chemical Activities. J Clin Endocrinol Metab. 2019 Nov 1;104(11):5393-5405. doi: 10.1210/jc.2018-01880. PMID: 31393563; PMCID: PMC6773459.
Wise, Amber; O’Brien, Kacie; Woodruff, Tracey (2011). Are Oral Contraceptives a Significant Contributor to the Estrogenicity of Drinking Water? Environmental Science & Technology, 45(1), 51–60. doi:10.1021/es1014482
Waugh DT. Fluoride Exposure Induces Inhibition of Sodium/Iodide Symporter (NIS) Contributing to Impaired Iodine Absorption and Iodine Deficiency: Molecular Mechanisms of Inhibition and Implications for Public Health. Int J Environ Res Public Health. 2019 Mar 26;16(6):1086. doi: 10.3390/ijerph16061086. PMID: 30917615; PMCID: PMC6466022.
Christofi, MD., Giannakou, K., Mpouzika, M. et al. The effectiveness of oral bovine lactoferrin compared to iron supplementation in patients with a low hemoglobin profile: A systematic review and meta-analysis of randomized clinical trials. BMC Nutr 10, 20 (2024). https://doi.org/10.1186/s40795-023-00818-6
Aktuna D, Buchinger W, Langsteger W, Meister E, Sternad H, Lorenz O, Eber O. Beta-Carotin, Vitamin A und seine Trägerproteine bei Schilddrüsenerkrankungen [Beta-carotene, vitamin A and carrier proteins in thyroid diseases]. Acta Med Austriaca. 1993;20(1-2):17-20. German. PMID: 8475673.
https://www.hormonesmatter.com/oxalate-a-potential-contributor-to-hypervitaminosis-a/
Shultz TD, Howie BJ. In vitro binding of steroid hormones by natural and purified fibers. Nutr Cancer. 1986;8(2):141-7. doi: 10.1080/01635588609513887. PMID: 3010251.
Eugster EA. Aromatase inhibitors in precocious puberty: rationale and experience to date. Treat Endocrinol. 2004;3(3):141-51. doi: 10.2165/00024677-200403030-00002. PMID: 16026110.
Feuillan P, Merke D, Leschek EW, Cutler GB Jr. Use of aromatase inhibitors in precocious puberty. Endocr Relat Cancer. 1999 Jun;6(2):303-6. doi: 10.1677/erc.0.0060303. PMID: 10731123.
https://www.healthline.com/health-news/forget-98-6-humans-now-have-lower-body-temperature-on-average-heres-why





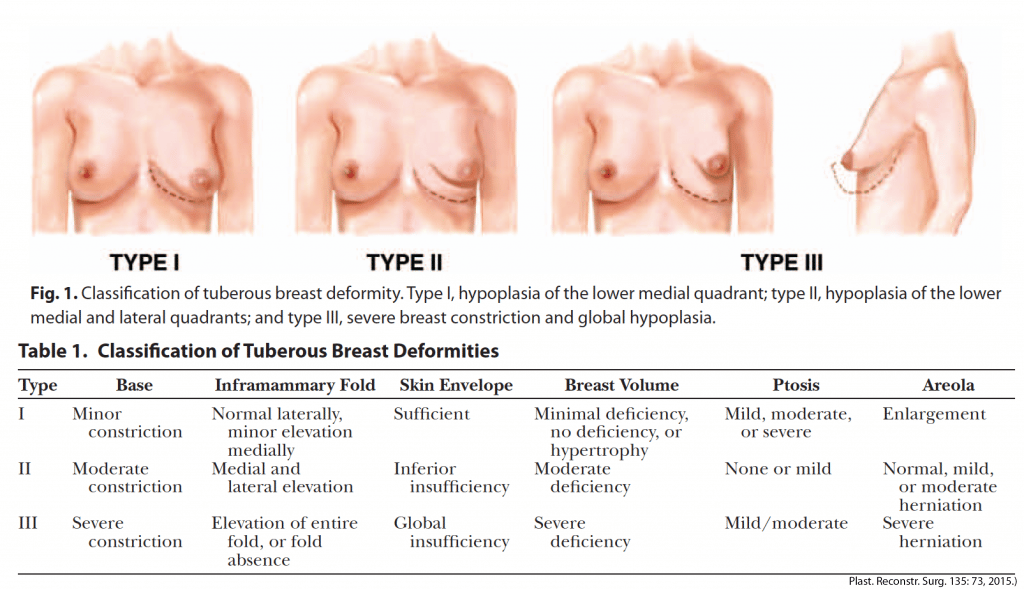


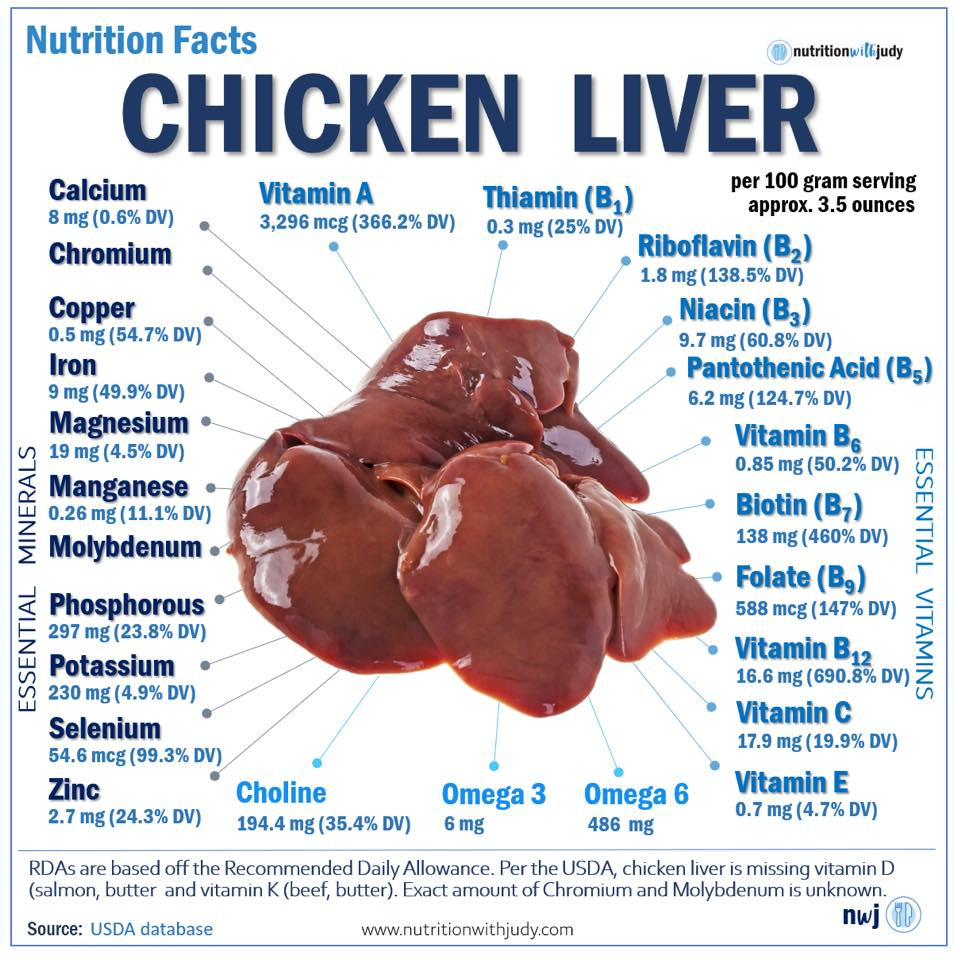
Hi kaya. I am a paid subscriber and just want to say I think your articles on health and nutrition are some of the most important content I’ve ever read on substack, or elsewhere. I really appreciate your work and it has informed my life.
hi kaya
I would like to ask you what do you think about fasting!?
just today I spoke with one of the world's leading experts on the topic. Doctor Filonov.
He claims to have treated thousands of people and freed them from hypothyroidism drugs.
come and see about it?
I'm asking because I respect your way of thinking and your insight.
Thank you!