Why Do Low-Fat and Low-Carb Diets Both "Work" For Diabetes?
And is one approach better than the other?
My Substack is a reader-supported publication. The full version of this article is available to paid subscribers ($15 per month or $99 for a yearly subscription). Paid subscribers get access to all my articles and podcast episodes in full.
Disclaimer: This content is not intended as treatment or support for any medical condition. Content for entertainment purposes only. Not medical or health advice.
“Ideally, things should make no sense until they make the right sense.”
- Ray Peat, PhD
Navigating the nutrition world as someone with type 2 diabetes, or as a family member of someone with type 2 diabetes, can be extremely exhausting considering that the various recommendations out there seem so insanely contradictory.
Some sources claim that a low-carbohydrate ketogenic diet with a heavy focus on fatty meats is best for diabetics and can lead to remission. Anecdotes from those following such diets show that they do in fact experience lower blood sugar levels, weight loss, and a general improvement in their diabetic symptoms.
Other sources claim that a high-carbohydrate low-fat vegan diet is the best for diabetics, and can similarly lead to remission. Similarly, there are many anecdotes of those whose diabetes seem to resolve on diets consisting of upwards of 80% of total daily calories coming from carbohydrates, mostly in the form of fruit.
Yet other sources bolster the benefits of “Mediterranean” diets in reversing diabetes, with similar positive anecdotes.
Could it be that the reason why such varied diets all seem to work for diabetics is that they are all arguably more nutrient-dense than the average highly processed Western diet, which is often built on staples such as white bread and chips? That is part of the answer. But there is a lot more to it.
Let’s not forget that apart from the above-mentioned “diabetes-busting,” diets the most common mainstream recommendation to combat diabetes is to just cut calories to lose weight. Even though the “weight loss at all cost by all means of restriction necessary” approach tends to worsen micronutrient deficiencies, in many diabetics, just losing some weight brings on improvements or even a remission of diabetes.
How could it be that such opposing approaches all seem to bring on remission in diabetics? Is it in fact because, as some like to claim, “we are all different”?
While there is a degree of bio-individuality from person to person, it is not like some of us are gazelles and others are rhinoceroses, lending our species-appropriate diets to be vastly different from person to person. We, as humans, are still all one species, and considering the makeup of our digestive tract and our nutrient needs, the species-appropriate diet that seems to be the most supportive for humans is a pretty homogenous omnivorous diet that doesn’t eliminate any major food group.
Some research suggests that red meat causes diabetes, other research suggests that it doesn’t. Some research suggests that saturated fat contributes to insulin resistance, other research suggests that it doesn’t. Some research supports the inclusion of more fibre for improving blood sugar balance, other research shows that zero-fibre carnivore diets work much better than high-fibre diets.
Are the disparities between the various diets that seem to bring diabetes to remission the great paradox of nutritional science that we will never be capable of explaining? No, they are not. There is an explanation that ties all of these pieces together and finds the nuance in all of it, and it is provided in this article.
And lastly, are all of these approaches, whether low-carb, low-fat, or calorie restriction, equally as effective at reversing diabetes and supporting overall health? Is it just about picking the one that will be the easiest for you to adhere to? Not quite.
While some of these approaches work to solve the underlying root cause behind diabetes (struggling to burn glucose well), others just sidestep the issue. Additionally, even though some of these approaches are better than others, at least as far as solving the root of the problem goes, none of the extreme approaches (such as low-fat veganism or keto carnivore) are optimal. Most of these contradictory approaches have aspects to them that are genuinely health-promoting. However, there are also aspects to all of these dietary ideologies that not only make them mostly unsustainable long term but can also lead to worse health outcomes in the long run.
Is there a way where we could take the best things from each one of them and take away the aspects that suck to create a better, more balanced approach?
Yes, there is! We can learn from all of these contradictory interventions to find a more optimal and more balanced way forward, one that is more supportive of long-term positive health outcomes.
To understand what this better, more balanced and more optimal way is, we need to first understand the aspects of the above-mentioned dietary approaches that make them “work” as far as remediating diabetes goes. This article will do just that, by analyzing these approaches, explaining why they “work,” evaluating what we can learn from each of them and summarizing all these learnings into an actionable guide at the end.
“It Ain’t Stupid if it Works?”
I understand that if you’ve dealt with diabetes or a pre-diabetes diagnosis and managed to reverse it through becoming a low-fat vegan, or through eating nothing but steak and butter, or simply by slashing your calories in half and starting a jogging habit, it can be easy to not care very much about why your approach worked. You’re just happy that it worked, that you probably feel better, that your blood sugar readings are lower, and that your doctor is finally off your case about your blood labs.
While it’s incredible that you were able to start feeling better and get your labs into a better place, it’s always important to ask:
Is this approach sustainable as far as long-term health goes?
Is this approach supportive of long-term metabolic health or is it actually destructive?
Is this current approach getting to issues at the core of the diabetic condition, or is it simply just reducing symptoms (while potentially silently worsening the underlying dysfunction in the background)?
Understanding the mechanisms through which these varied approaches produce symptomatic improvements (temporarily at least, as is the case with some of these approaches) will help us answer these questions.
In this article:
What is Type 2 Diabetes, Really?
Insulin’s Main Job Isn’t Making Cells Take Up Glucose
When Cells Struggle to Burn Glucose for Energy, Glucose Stays High in the Blood
This is Why Diabetics’ Cells Struggle to Burn Glucose
When Cells Struggle to Burn Glucose, High Blood Glucose Levels Become a Major Stressor on the Body
Curing Diabetes…With Sugar? (Dr. Kempner’s Rice Diet)
Dr. Kempner Did Not Have It All Figured Out
Dissecting the Rice Diet
What Made the Rice Diet Effective?
What Made the Rice Diet Ineffective?
Protein-Restriction, a Double-Edged Sword
High-Carb vs. Low-Carb for Diabetes
Solving the Problem vs. Side-Stepping the Problem
Cutting Out Macronutrients Can Backfire
When Keto Makes Things Worse
Does Fat Get a Pass a the Macronutrient That’s Okay to Cut Out?
Is There a Middle Ground?
Is Saturated Fat Worse For Diabetics?
What’s So Special About Dairy Fat?
What We Can Learn from Keto/Carnivore Diets
To Fibre or Not to Fibre, That is The Question
A Rarely Considered Risk of Keto/Carnivore Diets for Diabetics
Should Diabetics Restrict Their Calories?
What We Can Learn from All of These Different Approaches
A Practical Guide to Unite Contradictory Learnings
In Conclusion…
What is Type 2 Diabetes, Really?
To start, let’s make sure that everyone is on the same page as far as having a basic understanding of the mechanisms of action at the core of type 2 diabetes.
There are many misconceptions about what diabetes is and about what causes it.
Nearly everyone believes that diabetes and insulin resistance are caused by sugar. The mainstream explanation of insulin resistance is that as a “punishment” for eating too many carbohydrates, your cells get tired of responding to insulin’s signal to take up glucose and become insulin resistant. The misconception that carbohydrate consumption is what directly causes cells to become “desensitized” to insulin is why carbohydrates are so demonized.
However, as you will see later in this article, very high carbohydrate diets (high in refined carbohydrates such as white rice and white sugar) have been shown to induce a remission of diabetes. If we stick to the mistaken belief that carbohydrates themselves are the cause of diabetes, the above becomes a paradox. Unless we reexamine our preconceived beliefs about diabetes. Then the “paradox” starts making lots of sense.
Insulin’s Main Job Isn’t Making Cells Take Up Glucose
What a statement, huh? Insulin often gets painted as the hormone that simply pushes glucose into fat cells for storage. Many blood sugar “experts” like to claim that insulin is there to “save” the body from the “destructive glucose molecules” by “pushing them into fat cells for storage.” However, this is an extremely misleading explanation, one that seems to completely skim past the fact that glucose is our cells’ preferred fuel and not something that the body simply tries to “dispose of” as if it’s a poison.
Insulin has a far more complicated role, with its main functions consisting of:1
Turning off the body’s backup fuel generators when fuel comes in
Stimulating mineral retention
Creating more opportunities for some cell types to take up glucose
Let’s tackle the last point first.
Glucose enters cells through receptors called GLUT receptors. There are 14 known GLUT receptor types, which are numbered from 1-14. Of the 14 GLUT receptor types, only two have been identified to have a relationship with insulin: GLUT4 and GLUT12.2
Does insulin stimulate GLUT4 and GLUT12 to take up glucose? It does not. It stimulates the translocation of more of these receptors to the cell membrane, creating more opportunities for glucose to enter the cell.3 In other words, insulin doesn’t push glucose into the cell through the “doors” on the cell membrane. Insulin simply triggers the creation of more doors that glucose can use to pass into the cell freely as it pleases.
What about the other glucose receptors? Well, insulin doesn’t stimulate the translocation of GLUT1, GLUT2, GLUT3, GLUT5, GLUT6, GLUT7, GLUT8, GLUT9, GLUT10, GLUT11, GLUT13 and GLUT14 into the cell membrane at all. This means that all these other receptors take up glucose and fructose without the involvement of insulin at all.
Since of the 14 receptor types that take up glucose, the expression of only 2 is affected by insulin, we can see that insulin’s main role isn’t to “force cells to take up glucose.”
GLUT4, the receptor type whose translocation to the cell membrane is increased by insulin, is expressed mostly in muscle and fat cells. Since most people have a lot more muscle mass than fat mass, the increase in GLUT4 expression has more of a role in replenishing the glycogen stores of muscles and providing them with energy. Insulin doesn’t simply shuttle glucose into fat cells for storage as body fat. It makes it easier for muscle and fat cells to take up glucose to turn it into cellular energy (which they need to power all of their functions, maintain their structure and repair themselves), and only once these energy needs are met do fat cells convert excess glucose to body fat.
Now, what about the other roles? Let’s talk about insulin’s role in turning off the body’s backup energy generators.
In diabetics, high blood glucose is mostly caused by the liver overproducing glucose from non-carbohydrate sources.4 During periods of fasting (between meals), the liver helps to supply glucose to the bloodstream. This is necessary to have a steady supply of glucose for our brains and nervous system when we go a while without food. It keeps us alive.
When we eat food, insulin’s role is to signal to the liver: “Hey, fuel just came in. You can stop making sugar.” Insulin also tells our fat cells “Hey, stop releasing fat into the blood!” This blood fat is normally liberated into the bloodstream between meals to provide fuel for cells that have the option to switch from burning glucose to burning fat for energy, such as muscle cells.
By the way, dietary protein (such as steak) also stimulates the release of insulin.5
In insulin resistance, the body fails to respond to insulin’s signal telling it to turn off the backup energy generators even when fuel comes in. Even though fuel comes in, the body continues to liberate fat into the blood and the liver continues to produce more glucose out of protein and fat.
What causes this? Cortisol, the main hormone elevated under stress, can block insulin’s calls.6 Cortisol and insulin send opposing signals, and they play a sort of tug of war with one another.
Cortisol tells the liver: “Make more sugar.” It also tells fat cells: “Release fat into the blood!” Insulin tells the liver: “Stop making sugar” and tells fat cells “Stop releasing fat into the blood!”
When cortisol is too high, it can muffle the signal sent by insulin.
Cortisol can become chronically elevated by chronic psychological stress or by hypothyroidism, which decreases the clearance of cortisol. Type 2 diabetics have been found to have higher chronic cortisol levels than healthy individuals, with more diabetic complications being seen the higher their cortisol climbs.7 Type 2 diabetics also have high rates of hypothyroidism.8 The issue with hypothyroidism is that it often gets missed by doctors due to faulty lab testing, meaning that the majority (if not all) of type 2 diabetics might have some degree of it. I talk about this more in my hypothyroidism article.
Certain inflammatory mediators and the activation of certain inflammatory pathways, such as NF-κB, JNK, TNF-α, IL-6, and TLR4, can also block insulin’s signal. Factors that elevate these inflammatory mediators and activate these pathways include chronic underlying infections, gut dysbiosis, and high levels of body fat, especially if that fat consists of mostly polyunsaturated fat (such as that found in vegetable oils and conventional pork and chicken).910
Providing more and more insulin can eventually “outcompete” the cortisol signal. This is why giving type 2 diabetics insulin injections eventually drops their blood sugar. This, however, can also result in too steep of a blood sugar drop.
Those who are on their way towards developing type 2 diabetes often have chronically high insulin levels, as the body’s response to try and counter high cortisol levels. Hyperinsulinemia is what can lead to dark skin patches in diabetics, such as dark skin patches behind the neck or around the groin area. This is because insulin is an anabolic hormone that stimulates growth. When too much of it is being produced, it can cause rapid cell growth, creating these dark skin patches.11
In short, insulin resistance isn’t caused by the body getting “tired” of responding to insulin to punish you for eating carbs.
Insulin resistance is brought on by factors such as hypothyroidism, chronic stress, infections, the breakdown products derived from oxidized polyunsaturated fats, and high body fat levels, all of which increase the levels of cortisol and inflammatory mediators that block insulin’s signalling. As a result, the liver keeps making glucose and the fat cells keep releasing fat into the blood, while the ability of muscle cells to take up glucose is reduced. High levels of fat in the blood also interfere with the body’s ability to burn glucose for energy, which is something that the next section will discuss. This manifests as insulin resistance. Addressing these foundational pieces (for example, clearing underlying infections, reducing stress or addressing low thyroid function) is the key to addressing insulin resistance, and proof supporting this will be provided later in this article.
Insulin is not the bad guy, and when we do eat food and stimulate its release, it also helps cells retain important minerals that help to burn glucose for energy, such as potassium and magnesium.1213
When Cells Struggle to Burn Glucose for Energy, Glucose Stays High in the Blood
The cells of those who suffer from type 2 diabetes tend to have a hard time turning glucose into cellular energy. However, what’s worth noting is that the cells of those with type 2 diabetes are bad at extracting cellular energy from fuel in general, which is why diabetics tend to have high levels of not just glucose in the blood but also fatty acids, ketones and lactic acid.
While glucose can enter cells freely (as long as there are enough GLUT receptors on the cell membrane), if it fails to enter cellular respiration (which is the process through which glucose gets converted into cellular energy), it can flow back out of the cell and into the blood, maintaining high blood glucose levels. But why would glucose fail to enter cellular respiration?
Cellular respiration (the process through which cells turn glucose into cellular energy) is often inhibited at one or multiple steps in the respiratory chain in diabetics, or it happens at a rate that is far too slow.
When there are blocks along the respiratory chain, a few different things can happen to the glucose that enters the cell. If the block is at the level of the Krebs cycle, glucose will be converted into lactate instead of being burned for energy. This is why diabetics tend to have high lactic acid levels. When the respiratory chain is not moving as fast as it should, glucose metabolites can stay in the cell for too long, until they start “crumbling” into intermediates that can become AGEs (advanced glycation end products) and contribute to glycation (high HbA1C). Either way, in diabetes, glucose is burned at a rate that is far too slow and fails to be completely oxidized most of the time.
This is Why Diabetics’ Cells Struggle to Burn Glucose
Understanding the factors that inhibit glucose entry into cellular respiration and cells’ ability to fully turn it into energy will set the tone for the rest of the article and help explain the mechanisms of action behind the soon-to-be-discussed diets.
Now, what can cause glucose to fail to enter cellular respiration? This is where insulin becomes relevant.
The cells of diabetics tend to struggle to shuttle glucose towards cellular respiration because their cells fail to “respond” to insulin’s signal telling the liver to stop producing glucose, telling fat cells to stop releasing fat into the blood, and telling cells to express more glucose uptake receptors. As a result, diabetics have both high levels of blood fat and blood glucose.
High levels of blood fat can block the translocation of GLUT4 receptors.14 Additionally, if fats are over-supplied, many cells will end up burning fat for energy than glucose. Since any cell can only burn either fat or glucose at any given moment, if a cell is already burning fat, it won’t take up glucose.
High levels of adrenaline and cortisol also directly stimulate the release of fat from body fat stores into the blood.
In short, high levels of fat in the blood (either due to a diet that’s very high in fat or due to excess fat liberation into the blood by the stress hormones) will interfere with the burning of glucose for energy, as fat outcompetes glucose.
Glucose can also fail to enter cellular respiration when a cell has already taken up glucose for cellular respiration, but the process is happening too slowly or incompletely. In other words, it is impaired.
The main factors that can cause these impairments are:
Micronutrient deficiencies (vitamin B1, for example, is required for glucose to be burned for energy, and in a B1 deficiency, glucose gets turned to lactic acid instead)
Low levels of intracellular thyroid hormone, T3. T3 controls the speed of cellular respiration. (Micronutrient deficiencies, gut issues, liver problems and low-carb diets can lower T3 synthesis. High cortisol levels can deactivate T3. Polyunsaturated fats can prevent T3 from binding to its receptor, which would allow it to have an active effect on cells. Micronutrient deficiencies can lead to low levels of the carrier proteins needed to carry T3 to cells).
High levels of oxidative stress (Reactive oxygen species can deactivate the various parts of the cell involved in cellular respiration. Reactive oxygen species are formed most readily by metals reacting with polyunsaturated fats. The neutralization of reactive oxygen species depletes micronutrients involved in antioxidant defences).
Environmental toxins (Heavy metals, plastics and other chemical pollutants can deplete micronutrients, increase oxidative stress, interfere with thyroid hormone synthesis and cell entry, and downregulate the function of various cellular components involved in cellular respiration.)
Bacterial toxins (Toxins produced by bad gut bacteria, such as endotoxin, can upregulate the production of nitric oxide. Nitric oxide can block the mitochondrial complex at the bottleneck of energy production, complex IV. When these bacterial toxins reach the liver, they can impair the liver’s ability to detoxify estrogens and environmental toxins and to synthesize thyroid hormones).
Low levels of intracellular oxygen (Oxygen must be able to enter the cell, as it is the passing of electrons to oxygen during cellular respiration that facilitates the generation of cellular energy)
Excessive EMF and blue light exposure can similarly elevate blood glucose levels by negatively affecting cells’ ability to make energy, but this point is out of scope for this discussion.
When glucose becomes stuck in the blood (because cells can’t take it up), the body experiences osmotic stress. In other words, there is an imbalance between the amount of fluid in the blood vs. the amount of other compounds in it.
The body will send some of the glucose to the kidneys to dispose of it. As per the etymology of the Greek and Latin words “diabetes” and “mellitus,” the name of the disease roughly means “peeing sweet,” and sweet pee is a key sign of sugar loss in urine. As the body tries to dispose of excess sugar via urine, the glucose pulls more water into the kidneys. This is why diabetics pee a lot and are also thirsty a lot. However, frequent urination depletes micronutrients, such as potassium and B vitamins, which are needed for glucose metabolism.
When Cells Struggle to Burn Glucose, High Blood Glucose Levels Become a Major Stressor on the Body
When glucose stays high in the blood and the body is not able to burn it for energy, the body will also start sending glucose down pathways that dispose of it, such as the polyol pathway. However, this pathway depletes key nutrients (such as vitamin B1), and it creates intermediates that contribute to glycation (high HbA1C). Advanced glycation end products (AGEs) increase oxidative stress and the synthesis of various inflammatory cytokines. This pathway also depletes antioxidants, which can lead to more oxidative stress. This increase in oxidative stress and inflammatory cytokines can further inhibit cellular respiration. I talk about glycation, AGEs, and these pathways in more detail in my article “Eating Carbs Doesn’t Cause Glycation and High HbA1C.”
The numbing in the hands and feet of diabetics can be the result of inhibited energy production, oxidative stress and thiamine (vitamin B1) depletion, as thiamine is needed for proper nerve function.
The fatigue, hunger, slow wound healing and frequent infections experienced by type 2 diabetics are a direct result of inadequate cellular energy production, as adequate cellular energy (adequate levels of ATP) is needed to signal satiety, power the immune system, heal wounds, create hormones, and run every single other system in the body.
In summary, diabetes is a state in which the body struggles to burn glucose well. This inability and the consequent high blood glucose levels are not caused by glucose. They are caused by various stressors, as outlined above. Unfortunately, as the body becomes bad at burning glucose, the consequent chronically high blood glucose levels become a stressor themselves, in an awful feedback loop.
Curing Diabetes…With Sugar?
Since diabetes is often thought of as being caused by sugar, saying that sugar does not cause it might raise some eyebrows.
Now, going even a step further, me telling you that in some instances diets of up to 90% of all calories from carbohydrates have been seen to cure diabetes, might have your eyebrow flying off your forehead and hitting the ceiling.
But don’t just take my word for it. Read on, and you will be introduced to the work of someone who has demonstrated that this works.
Introducing… Dr. Walter Kempner and his diabetes-decimating invention…the rice diet.
I first learned about Dr. Kempner’s work from Denise Minger’s iconic article “In Defence of Low-Fat.”
After fleeing Nazi Germany for North Carolina in the 1930s, Dr. Walter Kempner continued his studies at Duke University where he developed an interest in kidney failure and malignant hypertension, a condition which often led to kidney failure.
At the time of his studies, there were no drugs available for those suffering from malignant hypertension, with the life expectancy of anyone diagnosed with the condition being only 6 months.15
After studying kidney cells under a microscope and seeing how they metabolize protein, carbs and fats, Kempner concluded that since what we eat dictates how hard the kidneys have to work (excreting waste products, amino acids, keto acids, etc), then by adjusting the diet of those with hypertension and kidney disease, it should be possible to salvage the kidneys.
His proposed diet? Under 20 grams of protein per day, only 2-3% of total calories from fat, a restriction of salt and fluids and permitting his patients to eat nothing but white rice, fruit juice (mostly orange juice for its citrate content), fruit and white sugar. In certain cases, he would also prescribe certain vitamins together with the diet, such as thiamine, niacinamide and the fat-soluble vitamins, knowing that white rice could lead to a depletion of these nutrients. Occasionally he would also allow his patients a slice of bread…as a treat.
Since Kempner had little faith in his patients’ compliance with the diet (recognizing its extreme and restrictive nature), he would often bully them into compliance and even created “rice houses,” which he treated as live-in clinics for his patients to stay at during the course of the diet. There, a rice chef would prepare their meals, and all of their moves would be strictly monitored by Kempner himself.
Kempner’s control freak tendencies and an often arrogant attitude alienated many of his peers, who already scrutinized his diet, suspecting Kempner of lunacy and having little belief that the diet would ever work. However, at a time when very little was known about malignant hypertension and kidney disease, rendering both of those diagnoses a death sentence, his “crazy” diet of 90% carbohydrates turned out to work…really well.
In his clinical experiments with seriously ill patients who had failed to respond to other forms of treatment, the diet would result in a decrease in blood pressure, decrease in heart size, disappearance of retinopathy, and an additional resolution of fatigue, headaches and pain in the majority of the patients on the diet.16
The patients who watched their health return after following the diet when no other approach worked and after being deemed as being on their deathbeds, became enthusiastic supporters of Kempner, getting other doctors interested in applying his approach. Again, they were all given 6 months to live and Kempner’s diet often gave them decades more.
The incredible success of the diet in curing hypertension propelled its extension to the treatment of other diseases, such as hypercholesterolemia, atherosclerosis, and…diabetes and obesity.
If Kempner’s colleagues looked at him sideways when he decided to apply his approach to hypertensive patients, you could only imagine how much they doubted its success in remediating the disease that was believed to be caused by sugar. And yet…
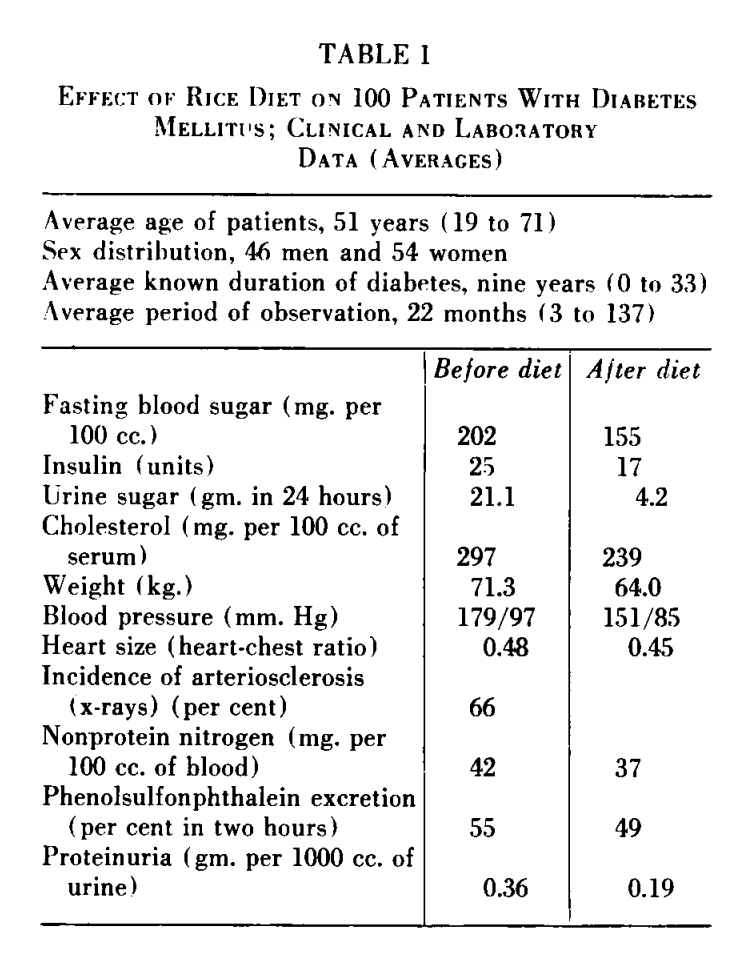
“A report is given on 100 consecutive patients with diabetes mellitus associated with vascular disease who were treated with the rice diet. […] The rice diet, which is a high carbohydrate, low protein, low fat, low sodium diet containing 565 to 570 gm. carbohydrate, 20 to 25 gm. protein, less than 5 gm. fat, and 70 to 120 mg. sodium per 2400 calories, was well tolerated. Average insulin requirements as well as average blood sugar levels decreased.” - Effects of Rice Diet on Diabetes Mellitus Associated With Vascular Disease17
On 2,400-calorie diets of over 500 grams of carbs daily, the majority of Kempner’s diabetic patients saw a decrease in blood sugar levels (on average by 47 points!), a decrease in the amount of insulin that they required, an average weight loss of 7 kg (15lbs), and far less sugar being lost in their urine. In 18 patients, insulin could be entirely discontinued after the diet.
And while it’s colloquially believed that sugar itself causes obesity, his diet also turned out to be an insanely effective weight loss strategy for obese patients.
Now, it’s important to mention that Kempner’s sugar diet was massively calorically restricted for these patients, only 1000 calories per day. However, many other diets were tried in the past with a similar massive caloric restriction, often not yielding results anywhere near what Kempner’s approach of fruit, sugar, and white rice produced. These were the results of Kempner’s patients:
“One hundred six massively obese patients, who each lost at least 45 kg, were treated as outpatients with the rice/reduction diet, exercise, and motivational enhancement under daily supervision. Average weight loss was 63.9 kg. Forty-three patients achieved normal weight. Men lost weight at a greater rate than women. Concomitant with weight reduction, there were significant decrements in blood pressure, fasting and two-hour postprandial blood glucose, serum triglyceride, and serum uric acid levels. […] The treatment of massive obesity is a perplexing problem for both patient and physician. The more conservative methods of caloric restriction and starvation (usually in hospital treatment) have not been met with great success. […] This has led to a proliferation of more drastic invasive approaches. […] All of these surgical procedures have definite associated morbidity and mortality. […] This report demonstrates that massive obesity can be corrected without resorting to invasive techniques and without either hospitalization or pharmacological intervention. The approach represents an extension of Kempner's earlier dietary programs.”18
Dr. Kempner Did Not Have It All Figured Out
I’m not mentioning Kempner’s diet because I think that he discovered some holy grail of nutrition. While Kempner has become a deity of sorts among vegans, with prominent vegan figures such as Dr. McDougall touting the rice diet as a magical cure-all, Kempner’s approach was far from perfect and it did not work 100% of the time.
The reason why I mention his work is because I think it serves as a “shock wave” to help break people out of the belief that sugar consumption itself is the root cause of the diabetic condition. If this was the case, Kempner would not have diabetics in remission on 2,400 calorie diets of white rice, white sugar and juice.
The important part comes now, which is understanding why his diet worked most of the time, and why it didn’t work for 15 out of his 100 diabetic patients, who actually saw their blood sugars and insulin requirements increase.
Dissecting the Rice Diet
Read on to see my analysis of what I believe to have been the beneficial aspects of the rice diet, and where the diet fell very short.