My Substack is a reader-supported publication. To gain access to all of my articles and podcast episodes in full and to allow me to continue devoting my time and effort to my writing, consider becoming a paid subscriber ($15 per month or $99 for a yearly subscription).
The full version of this article is for paid subscribers only.
Content for entertainment purposes only. Not medical or health advice.
In the holistic health world, there is a general view that all issues start in the gut. As such, it is often said that if you heal your gut, you will heal everything else.
While it is true that gut issues place a large, systemic burden on the body that will affect the function of every single organ system, most protocols aimed at healing the gut fail.
Not only do they fail at improving digestion long-term (or even short-term for that matter), but they also tend to fail at resolving all the other issues that are said to stem from the gut. Why? Because gut issues rarely start with the gut.
At the root of most (if not all) gut issues is a body that lacks the energy to keep the gut functioning as it should. Fixing this takes a much more foundational, big-picture approach and is not as simple as just popping a probiotic or prebiotic, as most gut-focused protocols would have you believe.
Even worse, most gut health protocols can end up exacerbating the issue further.
By promoting the elimination of endless foods and food groups (including sugars and carbohydrates as a whole), many gut health protocols are adding gasoline to the fire, propelling the dysfunction at the root of gut problems by making the energy deficit worse.
Additionally, the endless promotion of more fibre, more probiotics and more prebiotics is 99% of the time the exact opposite of what a delicate gut needs, especially when a pathogenic overgrowth is present, which tends to be the case when energy is deficient.
Our gut needs adequate cellular energy to function. Gut motility, preventing the migration of bacteria into the small intestine, maintaining the integrity of the gut lining, and producing digestive enzymes and stomach acid are all dependent on the gut (and the body as a whole) having enough cellular energy. Cellular energy is the energy that our cells make out of the foods that we eat, and that they use to power their function, structure, maintenance and repair.
Once gut issues emerge as a result of an energy issue, they tend to make the energy issue worse, creating a nasty feedback loop. For example, certain bacterial toxins produced by a dysbiotic or overgrown microbiome, such as endotoxin or enterotoxin, can block cellular respiration (the process of creating cellular energy out of the foods we eat), both directly and indirectly, by elevating certain inflammatory mediators. These toxins are produced at a greater rate when the microbiome is allowed to overgrow, which is more likely to happen when energy is low, with this low energy state making it easier for these toxins to pass through the gut lining and into the blood, reaching the liver and setting off the entire inflammatory, energy-inhibiting cascade. Metabolic issues worsen gut issues, and gut issues worsen metabolic issues.
As someone who has done all the “right” things in the past, including going gluten-free, dairy-free, sugar-free, grain-free, “processed food” free, taking all the probiotics, prebiotics and gut superfoods out there, only to end up with a bacterial overgrowth so bad that at one point the only food that my gut could handle was meat, I am writing this article for those who are sick of spinning their wheels chasing the next gut healing protocol or magic gut supplement, only to end up disappointed and get nowhere.
What helped me heal my gut was doing nearly the opposite of what most protocols would recommend. I increased my sugar intake (in the form of fruit, honey, juice, maple syrup and sugar), I reduced my fibre intake, I got dairy back into my diet, and I stopped avoiding grains. Doing so brought foods back into my diet that provide the calories, carbohydrates, and micronutrients needed to make the cellular energy that my digestive system needs to function. And while most “gut health” protocols would have you convinced that sugar/carbohydrates are the reason your gut health is poor, in reality, quite the opposite is true. How carbohydrates can actually help heal your digestion will be discussed later on.
My digestion is now better than ever, but the road to recovering my metabolism and my digestion wasn’t as simple as just eating all the foods and hoping for the best. It took me over 4 years to reintroduce some of my worst-offending foods (which, in my case, were tuberous vegetables such as sweet potatoes). Sometimes the road to eating more foods, healing the metabolism and improving our digestion is a little more winded and nuanced.
My goal is to demystify why the issue is there, and how to go about addressing it step-by-step. As you will see, the consideration of antimicrobials, probiotics or extra fibre, won’t be anywhere near the start of the list of what should be considered to get relief from gut issues, and for some, they won’t make the list at all.
Similarly to the structure of my “How to Cure Insomnia” article, the first half of this article will explain digestive issues and how they relate to poor metabolic function and excessive stress, while the second half will provide a guide on how to address specific digestive issues.
In this article:
My Gut Health Story
How to Spot Faulty Digestion
The Anatomy of the Human Digestive System (And Why Digestion Is a Whole-Body Process)
Gut Health is Energy-Dependent
Leaky Gut Is Caused by a Low-Energy State & Dysbiosis
How Carbohydrates Can Help to Heal the Gut
How to Heal Your Gut
1. Enjoy Your Meals and Eat Real Foods
Eat Tasty Meals in a Pleasant Setting
Cut Out Polyunsaturated Fats, Iron-Fortified Foods, and Foods with Non-food Additives
Cook and Properly Prepare Your Foods
Eat Enough & Often Enough, Address Deficiencies and Track Your Metabolic Rate
2. Avoid “Health Foods” That Are Ruining Your Health
Why Lactic-Acid-Containing Ferments, Probiotics and Blended Foods Can Worsen Gut Issues
How Fibre Can Be a Gut Health Menace
3. Consider Specialized Supplements and Antimicrobials
For SIBO
For Colonic Dysbiosis
For Constipation
For Diarrhea
For Low Stomach Acid
For Candida Overgrowth
For Gluten Intolerance
For Brain Fog
For Fat Malabsorption
For Parasites
4. Start Reintroducing Foods
The Connection Between Stressful Memories and Food Intolerances
My Gut Health Story
It wasn’t until I was 24 years old that I first realized that it’s possible to have a meal and not be incredibly bloated, uncomfortable and gassy afterwards. This realization came only once I ended up adopting an insanely restrictive diet of nothing but meat and some occasional raw milk, a diet that seemed like my only way out after my digestion got to the point where every meal left me in pain (I once nearly ended up calling the ER after eating some raisins and cacao nibs before bed, as I got woken up by bloating and pain so severe that I was convinced my intestine was going to rupture).
Spoiler alert - the restrictive carnivore diet wasn’t the solution, and it wasn’t the foods themselves that were the issue. The issue was much bigger than that.
But let’s back up, how did things get so bad?
While I must say that I never truly had perfect digestion when compared to others my age, my digestion was never worse than…when I was doing what most alternative health nuts would tell you is the ultimate gut-friendly diet.
Being bloated, experiencing excessive gas, dull pain in my stomach after meals, and hard-to-pass stools due to chronic constipation were a daily norm for me growing up. Factors as early on as possible definitely contributed to the development of these issues, including not being breastfed and being born via C-section, both factors that can interfere with the establishment of a healthy microbiome in a newborn. The problems with my gut were solidified by 4 years of nearly non-stop antibiotics that I ended up taking between ages 6-10 for endless infections, stemming without a doubt from childhood hypothyroidism, to which a stressful start to life and a somewhat chaotic upbringing contributed to without a doubt.
In my teens, I dieted excessively, partially influenced by wanting to attain the “beauty” standard of the 2000s, and partially probably as a way to deal with the interpersonal stressors and uncertainty that I was experiencing at that time in my life. The restrictive eating and social stress exacerbated my metabolic and gut issues further. Needless to say, my start to life wasn’t great as far as establishing a resilient gut and a healthy microbiome goes.
In my early 20s, I made the decision to start “eating healthy” and adopted the “paleo diet.” My diet became centered around eating heaps of raw vegetables, lots of greens, pastured meats, nuts and seeds, and the occasional berries, bananas or dried fruits. I cut out dairy, grains, legumes, and any pre-packaged foods, and started using stevia in place of any other means of adding sweetness to my food.
Little did I know then that a diet such as that is anything but healthy and is basically a recipe for worsening hypothyroidism and gut issues. Why such a diet is a gut health and metabolic disaster will be explained in more detail in further parts of this article but, for starters, part of the issue was that many of the foods I was eating weren’t super digestible, as in, they were full of fibre. Since humans can’t break down, absorb and turn fibre into energy (for example, we lack the enzyme cellulase which digests plant walls), we don’t derive any energy from fibre, and it passes through our stomach and small intestine undigested. However, what can digest fibre is bacteria. Since bacteria can feed on fibre, I was providing ample foliage for whatever microbes I housed in my gut to feed on. This wasn’t such a good thing, as my microbiome was overgrown and dysbiotic, and feeding it was amplifying my issues. I was also eating fewer cooked meals, as I started prioritizing salads and green smoothies, not knowing that cold meals take more energy to digest and are more likely to upset a sensitive digestive tract.1
I eventually started intermittent fasting, which really was just a way for me to avoid eating food throughout my entire workday, because my digestion had gotten so bad that it was easier to just not eat, to avoid having to be insanely bloated and gassy when at work.
The gut train-wreck that was paleo eventually led me to more restriction (carnivore), which provided me the relief I needed from bloating and gas (thanks to the complete elimination of fibre), but resulted in the worst constipation that I’ve ever experienced in my life (going weeks without a bowel movement), more food intolerances, and pushed me into a deeper layer of hypothyroidism - all of which are consequences of carbohydrate avoidance.
All in all, if there is such a thing as a digestive health rock-bottom, I think I’ve been near it, and can empathize with those who’ve had a similar journey, tried all the prebiotics, probiotics, and elimination protocols, and are at their wits ends. The good news is, I managed to pull myself out of there, as I finally put the pieces of my health puzzle together and realized that none of what I experienced was perplexing or random and that all my symptoms were just a manifestation of an impairment in energy metabolism, aka, how well my cells could make and use cellular energy. How all these pieces fit together, and what I did to get to a place where I can enjoy basically all foods now, will be explained throughout this article.
How to Spot Faulty Digestion
To make sure that we are all on the same page, let me start by describing what decent gut health looks like.
Healthy digestion is characterized by:
No bloating after meals. Bloating is not normal despite being common. There’s a trend of mainly fitness influencers on social media right now trying to “normalize” and “de-stigmatize” bloating. While I appreciate the sentiment, let’s not normalize dysfunction. Bloating isn’t normal.
Multiple well-formed bowel movements per day. Yes, daily. Yes, multiple. Many people believe that having a bowel movement every 2-3 days is normal or healthy. It is not, and it increases the risk of dysbiosis, bacterial overgrowth/SIBO, estrogen dominance, toxin reabsorption, and leaking of bacterial toxins into the blood. Ideally, each bowel movement should be a #4 on the Bristol stool scale.
No excessive gas or belching. Gas is a byproduct of bacterial fermentation. An excess of it can irritate the intestine, increasing the synthesis of inflammatory mediators, such as histamine, that negatively affect energy metabolism and increase gut permeability. Passing gas more than about 5-7 times per day should, in my opinion, get us curious. Belching is also a form of releasing excess gas and should be a red flag too if it happens anytime other than after drinking a carbonated beverage. Excessive belching can be a sign of SIBO or E.coli infection.
No brain fog after meals. Brain fog after meals can be a sign that we are overfeeding a pathogenic microbiome and the bacterial toxins released by it are irritating the intestine. This can also mean that the foods themselves are irritating the intestine. When the intestine is irritated, the body will synthesize more serotonin, which serves to draw water into the intestines to “flush out” the irritant. Excess serotonin can reduce blood circulation to the brain, resulting in brain fog.23 Brain fog can also be caused by an overproduction of bacterial lactic acid,4 which is produced as the end product of certain bacterial species (such as Lactobacillus) fermenting carbohydrates. Lactic acid is a metabolic burden, draining ATP (energy) and resulting in brain fog. Later parts of this article will go deeper into issues with lactic acid.
Not experiencing any of the following: Intestinal cramps, heartburn, extreme hunger immediately after meals, constantly feeling full, never feeling full, diarrhea, bloody stools, vomiting, or any sort of abdominal pain.
Additional signs that digestion is off can include dandruff, acne, skin rashes/eczema/psoriasis, mood swings, depression/mood disorders, post-nasal drip, constant congestion, tinnitus, allergic reactions, lower back pain, high cholesterol levels, bad breath, white, coated tongue and trouble sleeping. While these symptoms aren’t overtly digestion-related, they can be quiet signs that digestion is off.
Ideally, we shouldn’t really feel our digestion as it happens. After we eat, our gut shouldn’t distend, we shouldn’t feel stuffed or heavy, and we shouldn’t be feeling gas building up, hearing rumbling and splashing, or feeling any discomfort or an urgent need to use the bathroom. Our digestion should just happen in peace.
Digestion Is a Whole-Body Process
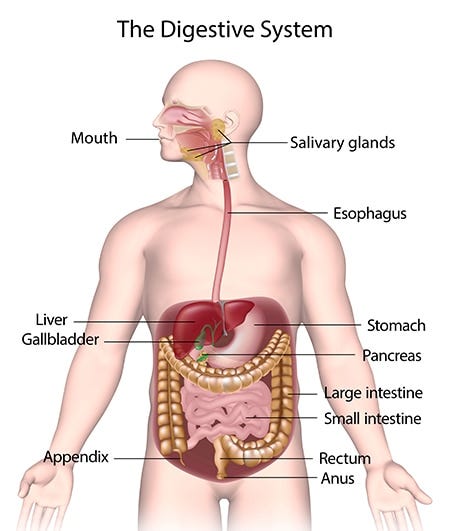
Now, just as important as understanding what good digestion looks like is understanding our digestive tract and its anatomy. I already went into this topic in depth in my podcast episode “What are humans meant to eat?” where I cover which foods are more vs. less appropriate for us based on the structure of our digestive system.
Understanding the anatomy of our gut, as well as all the hormones and organs that are involved in digestion, ones that commonly aren’t even considered part of the digestive system, will help to illustrate the complexity of the issue and show why suggesting that just taking a probiotic should be enough to “fix the gut” is ludicrous.
Some say that digestion starts in the mouth, but digestion actually starts with our mind and senses.
Just smelling or seeing food that we find tasty is enough for the body to start producing enzymes and hormones that prepare it for digestion. This is why eating foods that we find tasty is the first step towards improved digestion. You know when you see or smell something tasty (such as this picture of lasagna 🤤) and start to salivate? That’s your mouth releasing digestive enzymes.5
Even just thinking or talking about a tasty meal (without even smelling, seeing or tasting it!) increases the release of a hormone called gastrin, which increases stomach acid.6 Similarly, just the taste, smell or anticipation of eating something sweet can stimulate the pancreas to release insulin.
“Cephalic phase insulin release is the consequence of sensory stimuli from food (sight, smell, taste, proprioceptive, and tactile stimulation) acting on receptors in the head and oropharynx prior to the direct stimulatory effects of glucose on b cells (Berthoud et al., 1980; Powley, 1977).”7
In the mouth, enzymes released in our saliva jumpstart the digestion of carbs and fats, sanitize our food, and protect us against fungi like Candida. This is part of the reason why chewing our food well is so important.
If we chew bread, potatoes or another starch for a while, it will start tasting sweet. This is because amylase, an enzyme present in our saliva, starts breaking the starch down into separate glucose molecules. Humans produce at least 3 times as much amylase in our mouths compared to our closest relatives, the great apes, showing that starch has been a major constituent of our diets from an evolutionary perspective. Amylase also helps to dissolve and digest any starch that might get stuck to our teeth where we can’t reach it, effectively acting as a “biochemical toothbrush.”8 Lingual lipase is another digestive enzyme released in the mouth, this one responsible for kickstarting the digestion of fats.
The enzyme lysozyme and the protein lactoferrin are other important constituents of saliva. These two are antibacterial and part of our immune system. They help us kill any pathogens that might be contaminating our food (and prevent cavities too).9 Histatins are a family of over a dozen peptides released in our saliva, which kill bad bacteria in our food and protect the mouth against the overgrowth of fungi such as Candida.10 Proline-rich proteins in saliva, such as the protein IB-5, bind to certain anti-nutrients and plant compounds that can be toxic in large amounts to prevent their absorption and protect our digestive system against them.11 These are just a few of the many constituents of our saliva that help us digest foods.
All in all, properly chewing foods and allowing them to mix with saliva is paramount to good digestion. However, psychological stress and low metabolic function (as indicated by a waking core body temperature lower than 36.6 degrees Celsius/97.9 degrees Fahrenheit, a resting heart rate below 80, and cold hands and feet) interfere with the production and release of digestive enzymes and protective proteins in our saliva.121314 This means that if someone’s in a stressed, low metabolic state of energy shortage, their saliva won’t be as good at digesting and sanitizing their food. This is why poor digestion, bacterial overgrowths and fungal issues can’t be permanently fixed outside the context of fixing the metabolism! More on how the function of the digestive tract is dependent on the body’s ability to make and use energy will be covered in the next section.
Once we thoroughly chew our food, the saliva-food mixture passes from the mouth through the esophagus and into the stomach. While the breakdown of carbohydrates and fat starts in the mouth with the help of amylase and lingual lipase, the breakdown of protein starts in the stomach, with the enzyme pepsin. The release and optimal function of pepsin is dependent on the stomach maintaining an acidic pH,15 as stomach acid is needed to convert pepsinogen, the building block for pepsin, into pepsin. When the lining of the stomach is able to release adequate amounts of hydrochloric acid, it’s able to maintain its pH at the optimal, very acidic pH of 1-3 in its fasted state.
Various stressors (which results in an energy deficit) and hypothyroidism (which is an energy deficiency) interfere with the release of stomach acid.1617 The production of stomach acid is also dependent on adequate levels of certain nutrients, such as zinc, potassium and chloride.
“More recent investigations have confirmed a reduction in gastric acid output following exposure to stress induced by a large variety of stimuli including restraint, rotation, high ambient temperature, unpredictable electrical shocks, endotoxin, or ionizing radiation.”18
The contents of the stomach then pass into the small intestine. The speed of gastric emptying, and the ability of the sphincters connecting the esophagus and the stomach and the small intestine and the stomach to open and close with ease, allowing food to flow down and preventing it from coming back up, are under the control of the nervous system and, as all other parts of digestion, are energy dependent.
The small intestine is arguably the most important part of the digestive system. It is also where many things can go wrong, and where most issues tend to take place. In the small intestine, carbohydrates, fats and proteins are broken down into their simplest forms, absorbed by the intestinal wall, and transported into the bloodstream. The small intestine is also where the absorption of most vitamins and minerals takes place. Proper digestion in the small intestine is dependent not only on the integrity of the small intestine, but also on the cooperation of other organs, including the pancreas, the liver, and the gallbladder. Hormones produced by the small intestine, such as secretin, gastrin and cholecystokinin, influence the cooperation of these organs. The function and structural integrity of the small intestine are largely affected by hormones, such as cortisol, and are heavily dependent on the proper function of the nerves in the gut.
“Most of the processes that convert the food into suitable forms that the body can use happen in the small intestine, where the nutrients are absorbed.”19
In response to secretin, the pancreas releases:
Bicarbonate, which neutralizes the acidity of the food mixture that flows from the stomach into the small intestine.
The digestive enzymes amylase, lipase, and proteases (trypsin and chymotrypsin). These enzymes help to break down various proteins, sugars and fats.
The small intestine itself also releases a well-known enzyme, called lactase, which helps to digest lactose - the main protein in milk. An injured small intestine can struggle to release enough lactase, resulting in dairy intolerance.
The pancreas’ ability to release enzymes is decreased in hypothyroidism.20 The activity of the various enzymes (including lactase) is enhanced by thyroid hormone, whereas cortisol suppresses the activity of digestive enzymes.21
The presence of fat and protein in the small intestine causes the small intestine to release a hormone called cholecystokinin, which triggers the gallbladder to release bile (produced by the liver), into the small intestine. Bile emulsifies dietary fats to make them absorbable, but it also has many other roles in the small intestine. Bile is antibacterial22, prevents the migration of microbes from the large intestine into the small intestine, reinforces the structure of tight junction proteins (aka, helps to “seal up” a leaky gut, more on that soon),23 and helps to absorb fat-soluble vitamins such as vitamin A, E or K2. Excess estrogen can inhibit the flow of bile and its ability to leave the liver, interfering with the digestion of fats and fat-soluble vitamins.24 Most of the bile acids are reabsorbed at the end of the small intestine.
The last steps of digestion take part in the large intestine (colon). In a healthy digestive system, the large intestine is the only place where a large number of bacteria should live. While all the organs in a human body have some degree of a microbiome, the only place in our digestive tract where a relatively large microbiome should live is the large intestine. The small intestine should ideally be relatively sterile.
“The human colon has the largest population of bacteria in the body (in excess of 1011 organisms per gram of wet weight), and the majority of these organisms are anaerobes.”25
In a healthy digestive system, ideally, no or a very small portion of carbohydrates, fats and proteins should pass to the large intestine, as they should be absorbed in the small intestine. The only components of food that should pass in relatively large amounts into the large intestine are water and fibre. Water is mostly reabsorbed in the large intestine, and fibre provides the substrate for the microbiome of the large intestine to feed on.
Humans are hindgut fermenters. Our microbiome (our digestive bacterial farm) is located at the very end of our digestive tract, behind the stomach and small intestine. This design separates us from foregut fermenters, such as cows, sheep and leaf-eating monkeys.
As the name “foregut” implies, these animals’ microbial farm is located at the very start of their digestive tract, in their stomach. The stomachs (rumens) of foregut fermenters are alkaline as opposed to acidic, to create a hospitable environment for bacteria. As mentioned earlier, in contrast, human stomachs are acidic to kill off any bacteria.
In foregut fermenters, their bacterial farm gets to feed first, and only then do they get to digest and absorb the products of that bacterial fermentation, once they pass from their stomachs into their small intestines. As a result, even though a cow eats grass, it doesn’t directly feed on and derive energy from the grass. The bacteria in its stomach ferment the grass, turning it into mostly fats and some sugars and proteins, and the cow then gets to digest and absorb those fats, sugars and proteins, and use them for energy. For these animals, having a massive microbiome is absolutely essential, as it’s actually their bacterial farm that creates their food for them.
The human digestive system is the reverse of that. Our first major digestive organ is a stomach built for breaking down proteins, and the second one is a small intestine designed for further breaking down and absorbing fats, sugars, proteins, vitamins and minerals. As mentioned earlier, the small intestine is meant to have close to no bacteria living in it, to prevent us from having to compete against bacteria for the absorption of micronutrients, sugars, fats and proteins. Our bacterial farm gets to exist at the very end of our digestive tract, in the large intestine, indicating that, unlike cows or sheep, the fermentation of fibres is not a significant way in which humans are meant to get their energy needs met.
Additionally, our large intestine, contrary to its name, is actually quite short compared to other omnivores. For example, relative to our size, our large intestine is much shorter than that of other omnivorous animals, such as pigs. Relatively, our large intestine is somewhat between that of a dog and a pig in proportion.
“Comparisons of digestive tract anatomy. It can be seen that the human digestive tract is relatively small. Compared with that in the pig, an omnivore that is often regarded as a model for humans, the human large intestine is much reduced. The dog intestine is capacious but relatively short. The human large intestine is also small compared with anthropoid apes, here illustrated by the orangutan.”26
This is relevant as the relatively small size of our large intestine further illustrates that fibre is not meant to be a key component of our diets and that eating too much of it can have consequences. Don’t get me wrong, this isn’t to say that fibre doesn’t have a place in a healthy diet, as it definitely does. Fibre helps us bind to and excrete old estrogens, environmental toxins, bacterial toxins, and old bile, and those who consume fibre tend to have lower estrogen levels. However, eating heaps of raw vegetables and salads for every meal gives our digestive system the reverse of what it’s designed to handle. Since our microbiome is housed in such a short large intestine, overfeeding it can cause it to overgrow to a degree beyond our large intestine’s ability to accommodate it, which can create issues such as excessive gas production, bloating and the spilling of bacteria into the small intestine (SIBO).
In our digestive tract, as shown in the image above, the large intestine actually curves upwards, climbing above the small intestine and just below the stomach. Many cases of bloating and distention might thus be mistaken as issues with the stomach or SIBO, but they might just be a symptom of overfeeding the colonic (large intestine) microbiome with too much fibre. It’s important to feed our microbiome, but it’s even more important to not overfeed it.
Since food takes some time to make its way from the stomach, through the small intestine and to the large, bloating after a meal can sometimes be caused not by the meal that was just consumed, but by a previous meal getting pushed down into the large intestine. This is especially likely if the earlier meal contained lots of fibre.
The environment of the large intestine should also be mostly anaerobic (lacking in oxygen) to support the presence of commensal (good) bacteria and prevent the proliferation of bad bacteria. This part is important as in many disease states, especially in cases of energy deficiency, the gut can become more aerobic (oxygen-rich), making it easier for bad bacteria to thrive, and harder for good bacteria to make it.2728 This is in part due to the reduced production of CO2 in those who struggle to produce enough cellular energy (CO2 is a byproduct of energy metabolism). The way in which a person breathes can contribute to this too. Hyperventilation, which is common in those who are stressed and hypo-metabolic and often happens unconsciously, can make the gut too oxygen-rich by exhaling too much CO2.
“Hyperventilation, breathing excessively and causing too much carbon dioxide to be lost, is similar to being in the presence of too much oxygen. […] Hyperventilation is present in hypothyroidism, and is driven by adrenalin, lactate, and free fatty acids.” - from the article: “Altitude and Mortality” by Dr. Ray Peat, PhD.
“Inhalation of oxygen, leading to hyperoxia, has been extensively investigated in preclinical studies to understand its impact on the gut microbiome. […] “Exposure to hyperoxia, both in neonatal and adult mice, has been linked to a marked expansion of oxygen-tolerant microbes, particularly pathogenic Proteobacteria, Enterobacteriaceae, and Staphylococcus. Concurrently, it has led to a decrease in the relative abundance of oxygen-intolerant microbes like Bacteroidetes and Muribaculaceae, as well as Lactobacillus in mice.”29
As you can see in the image above, dysbiosis is characterized by the decline of the anaerobic bacteria, with the severity of dysbiosis increasing the more that the levels of anaerobes decrease and the more that the levels of aerobic bacteria and fungi increase. Again, the gut becoming more aerobic (oxygen-rich) is downstream of metabolic issues, which are at the core of gut issues.
Taking probiotics hoping that they will colonize the gut and fix your digestion when the gut is too aerobic to keep these bacteria surviving and thriving (due to an energy deficiency or due to hyperventilation, both of which result in a loss of CO2) is like trying to convince a middle-class golden retriever family to move into an abandoned shack. It’s not going to happen.
If we have a good balance of the good bacteria in the large intestine (which is largely dependent on the health of the large intestine, which is downstream of good metabolic health), these bacteria will feed on and break down fibres (especially soluble fibre, the type found in fruit or potatoes) into fatty acids, such as butyrate and propionate. These fatty acids protect us against bad bacteria, keep the gut more anaerobic (oxygen-poor) and help us maintain a healthy gut lining.30 The bacterial populations in our large intestine fluctuate in response to the foods that we eat.
An honorary mention also goes to the migrating motor complex.31 The migrating motor complex (MMC) is not a physical part of the digestive system (as in, it is not a distinct physical entity, in the way that an organ, such as the liver, is), but it plays an important role in digestion. The migrating motor complex is a pattern of movements throughout the digestive tract. It is a series of contractions that start in the stomach about 90 minutes after a meal and move down the length of the small intestine. The goal of these contractions is to clear away bits of old food, bacteria and dead cells, in order to clean the intestinal tract and make sure that bacteria from the large intestine don’t migrate up. In this way, the migrating motor complex helps to prevent conditions such as SIBO.32
“One important host mechanism limiting the proliferation of organisms in the small intestine is gut motility. In humans, the well defined phase III activity of the interdigestive motor complex (MMC) occurs every 84-112 minutes and migrates down the upper intestinal tract with a speed of about 6-8 cm/minute. The caudate moving band of intense contraction during phase III has been called the 'intestinal housekeeper.’ The contraction moves digestive contents rapidly down the small intestine. At the port of entry, gastric acid destroys most of the organisms from the outside environment. In the upper intestine this 'housekeeper' mechanism gets rid of microorganisms that managed to escape the gastric acid barrier, and thus prevents stagnation and bacterial overgrowth. The other important role of this movement is to maintain an appropriate distribution of the endogenous enteric microflora and to prevent migration of organisms from the colon.”33
The migrating motor complex process takes anywhere from 90 to 120 minutes, which is why it’s best to have meals no closer in frequency than every 3 hours. Frequent snacking, such as every hour, or drinking too much water in between meals, can stop the contractions of the migrating motor complex.
The migrating motor complex is under the control of the enteric nervous system. High cortisol levels can stop the migrating motor complex from taking place, or weaken the contractions.34 Hypothyroidism can also weaken the contractions of the MMC and slow the movement of debris through the gut.35 These go hand-in-hand as hypothyroidism results in higher cortisol levels, by decreasing the clearance of cortisol and lowering the body’s sensitivity to cortisol. Slow gut motility, leading to constipation and bacterial overgrowths, is typically caused by hypothyroidism.
Additionally, the ability of the nerves to transmit electrical signals that control the contractions of the migrating motor complex is dependent on certain nutrients, notably the B vitamins, especially B12 and B1, and minerals, such as calcium and magnesium. In general, peristalsis (the movement of food through the digestive tract) is largely dependent on the proper function of the nervous system and on having enough of these vitamins and minerals. Deficiencies of B1 and B12 can sometimes result in a “paralysis” of the digestive tract.3637
All in all, I hope that this section has made it clear that simply taking a probiotic or drinking a green juice will not be enough to fix a gut in a bad state as:
Proper gut health is dependent on the correct function of multiple organs outside of the digestive tract, including the liver, gallbladder and pancreas.
Digestion begins in the mind, before we even start eating, and how excited we are for our meal, how appealing we find it, and how relaxed we are prior to mealtime will all affect the secretion of digestive juices and enzymes.
Our levels of sex hormones and adrenal hormones can affect our digestive tract’s ability to secrete and utilize digestive enzymes, release bile, and move food along.
Our ability to create enzymes and gastric juice, and maintain gut motility is dependent on our nutrient reserves.
All of the above influence and are influenced by how well our body is able to create energy out of the foods that we eat. In a state of energy deficiency (such as hypothyroidism), digestion is slower, and the gut can become more aerobic and inhospitable to good bacteria.
Digestion and Gut Health Are Energy-Dependent
In the previous section, I mentioned the importance of energy more than once. If you’ve been reading my Substack for some time now, you are probably familiar with the view presented in the work of Dr. Raymond Peat, Dr. Albert Szent-Györgyi and others, one that I try to explain in simpler terms here, and one that basically views all disease states as a manifestation of an energy shortage in the body and the body having to make concessions.
This section is for those who might be new here or those who simply haven’t had digestion explained to them in terms of its dependence on energy metabolism. The goal here will be to explain why cellular energy is so important as far as gut health goes, and how certain hormones, including thyroid hormone, affect cells’ ability to make energy.
First, what is energy? Many think of the foods that we eat as energy, or the calories in them as energy. However, foods themselves, or their calories, are not energy. Foods, the right foods at least, provide the building blocks that our cells use to make energy, but they aren’t energy in and of themselves. The energy that our cells use is called ATP, adenosine triphosphate. This is the energy that our cells create out of the foods that we eat, given that they have the right cofactors to do so. The bulk of ATP is created in the mitochondria.
While we need to have enough calories coming in from food to be able to make ATP, we also need micronutrients for this process, and we need the appropriate proportions of the three macronutrients (protein, carbohydrates and fat). For example, excessive reliance on fat burning can actually “clog” mitochondrial complex I, creating a bottleneck in energy creation.
ATP can be thought of as little batteries that power the function, structure, maintenance and repair of all cells, including those of the gut. Since every single one of our organs is made up of individual cells and all the functions of our organs are powered by the individual cells that make up these organs, the function of our organs and all other systems in the body is dependent on those organs having enough ATP.
The rate of ATP creation is dependent on the active thyroid hormone, T3, entering our cells. When we don’t produce enough of the active thyroid hormone and/or if thyroid hormone fails to enter cells at an adequate rate, we can’t produce enough energy. As such, our body has to make concessions, deprioritizing or slowing many of its functions. Basically, when ATP isn’t abundant it has to get rationed. This is called hypothyroidism. This is why hypothyroidism is characterized by bodily functions that happen inadequately, incompletely, or slower than normal. Some symptoms that show that the body is lacking energy and is making concessions are a slow heart rate, a lower than optimal body temperature (and the resulting cold extremities), hair that doesn’t grow as fast/is falling out, constipation, decreased immune system function, troubles focusing, fat gain (because the body doesn’t have enough thyroid hormone to turn food into ATP, so it stores it as fat instead), etc. Hypothyroidism (energy shortage) is at the root of most gut problems and the reason the gut becomes hospitable to pathogenic bacteria, fungi or viruses.
“Gastrointestinal motility and serum thyroid hormone levels are closely related. […] Hypothyroidism prominently reduces esophageal and gastric motor activity and can cause gastrointestinal dysfunction.”38
“Altered gastrointestinal (GI) motility is seen in many pathological conditions. Reduced motility is one of the risk factors for development of a small intestinal bacterial overgrowth (SIBO). Hypothyroidism is associated with altered GI motility. […] SIBO is common in patients with hypothyroidism.”39
“Just as gastrointestinal functional diseases affect the thyroid, so thyroid disfunction affects the structure and function of almost all parts of the gastrointestinal tract. Hypothyroidism has frequently been associated with various gastrointestinal manifestations, including constipation, bloating, flatulence, atrophic gastritis, ileus, atony and dilatation of the oesophagus, stomach, gallbladder, small intestine and colon. Characteristic intestinal hypomotility in a severe hypothyroidism may progress to intestinal pseudoobstruction, paralytic ileus and megacolon.”40
The reduced immunity, resulting from this energy shortage that prevents the immune system from operating at full capacity, makes it easier for pathogenic bacteria to take residence in the gut.4142
“Hypothyroidism has a detrimental effect on the immune system, which may predispose patients to infection.”43
In turn, pathogenic bacteria can create toxins that block the function of thyroid hormones, create toxins that block cellular respiration, increase inflammatory mediators, and/or prevent thyroid hormone re-uptake.44
Going back to the concept of energy, the body’s ability to create thyroid hormones and to create ATP is dependent on the availability of many nutrients.
The various steps of ATP generation are dependent on a variety of nutrients, including magnesium, zinc, B1, B3, B5, B7, copper and iron, among others. The creation of thyroid hormones is also dependent on various nutrients, such as selenium, iodine, and certain proteins, such as tyrosine.
Oxygen needs to be able to enter cells in order to create ATP. CO2 helps to get oxygen into cells. Excessive reliance on fat burning for energy, as opposed to glucose burning, produces less CO2 and creates less ATP per molecule of oxygen.45
Various factors can interfere with the process of ATP generation, either directly or indirectly, including certain hormones and neurotransmitters (such as excess estrogen, cortisol, serotonin or histamine), bacterial or fungal toxins that make it into the blood and reach the liver, nitric oxide, heavy metals, and unsaturated fats that get incorporated into cell membranes. Women tend to be more prone to food intolerances and digestive issues than men, and higher estrogen levels in women (which increase serotonin, histamine, and cortisol, and lower thyroid function) are in part what’s to blame for that.
“In a study of self-reported illness from foods and chemicals among 490 male and female undergraduate students at the University of Arizona, 24% of the subjects reported at least occasional illness from one or more foods. The food-intolerant group was 80% female.”46
High levels of the adrenal hormones, such as cortisol and adrenaline, divert blood flow away from the digestive tract4748 to bring more energy to the nervous system, brain and muscles (to prepare for the fight or flight response). With diverted blood flow, fewer nutrients and less oxygen are brought to the digestive tract, interfering with its ability to make energy. The adrenal hormones also promote excessive fat burning and excessive liberation of fat into the blood. Excessive burning of fat for energy results in more oxidative stress49 (which, long term, interferes with the process of energy generation), and creates less ATP per molecule of oxygen. In hypothyroidism, the body produces more of the adrenal hormones (as it loses its sensitivity to them), and their clearance is impaired,50 meaning that those who are hypothyroid have higher levels of cortisol and adrenaline.
To put all of the above in simpler, more digestible terms:
The gut needs adequate ATP (energy) to function as it should.
In a state of low metabolic function, the gut doesn’t get enough ATP.
Chronic stress (whether psychological or biological) interferes with the gut’s ability to make and use all the energy that it needs, thus interfering with every aspect of digestion.
A state of low metabolic function is synonymous with chronic stress, as any person who’s hypothyroid (in a state of energy shortage) will have higher levels of cortisol and other adrenal hormones.
Of course, gut issues propose an interesting challenge.
We need to be able to digest our foods and assimilate their nutrients to create cellular energy. At the same time, a low energy state interferes with the body’s ability to make enough stomach acid, make and release enough digestive enzymes, keep the migrating motor complex functioning to prevent bacteria from growing into our small intestine and stealing our nutrients, and in general, digest and absorb foods and nutrients. Additionally, high cortisol levels cause the wasting of nutrients such as zinc, magnesium and the B vitamins.
You need to address the low energy state to digest better, but you also need to digest better to get the building blocks that you can make energy out of in the first place. It’s all a bit of a pickle.
One of the first steps in addressing such a pickle should be choosing foods that can feed us without feeding the overgrowth/dysbiosis, to get enough of the calories and nutrients to keep the stress hormones down and thyroid function up, as under-eating puts us in an energy-deficient state that makes us prone to bacterial, parasitic and fungal overgrowth in the first place. How to do so will be addressed in the latter parts of the article which will go into how to actually start addressing a damaged gut.
The most important takeaway from this section is understanding the importance of energy and the connection between stress (both psychological and biological) and energy, as far as proper gut function goes, including conditions such as low stomach acid, constipation, SIBO, heartburn, and leaky gut.
Leaky Gut as a Manifestation of a Low-Energy State & Dysbiosis
Understanding leaky gut from the perspective of how it relates to energy should help to really illustrate the issue with how many gut protocols approach the issue of leaky gut.
The image that gets painted in our heads of what a leaky gut is, is akin to imagining a wall with a crack in it. If we just “patch up” this hole, our gut will be “sealed,” and we won’t have to deal with the pesky issue of toxins and undigested foods from our gut passing over into the blood and causing an inflammatory cascade.
“All your issues are due to leaky gut, and once you seal your gut, your health problems will go away” - preach many in the health world. Sounds simple enough, doesn’t it?
Except our bodies aren’t made out of Lego blocks where something exists in a binary state of either broken or not broken.
The integrity of the gut exists in a fluid state, dependent on energy availability. The degree to which the gut is permeable or not permeable is dependent on the level of stressors to which the body is exposed and energy availability at any given moment. As such, it’s not possible to simply “seal” the gut as if you would a crack in the wall without addressing energy issues in the body as a whole.
For example, experiencing a psychological stressor (such as an argument or taking a test)5152 will cause the lining of the gut to become permeable (leaky). Acute stressors, such as taking a test, can increase gut permeability for a few hours, while chronic stressors can cause the permeability to be chronically increased. For example, one study found that relationship stress can chronically cause the gut to be permeable, with those in hostile relationships having more leaky guts and, as a result, increased levels of bacterial toxins in the blood.53
“Elevated stress and cortisol levels increase gut permeability, increasing the number of digestion components traversing the gut wall barrier and entering intra- and extra-cellular spaces in the host.”54
This is in part because when we enter fight or flight, as mentioned earlier, the body will partition energy away from digestion, and towards providing the brain and muscles with additional oxygen and energy. This is an adaptive strategy. When our life is in danger, the body wants to make sure that the brain and muscles have as much energy as possible, so that we can think quickly on our feet and swiftly escape the perceived threat.
However, when we are constantly in a state of energy deficiency, the body will consistently be forced to divert some of the energy available to it towards more immediately important functions (such as keeping the nervous system running and keeping the heart beating), while the gut gets deprioritized. This is because, while digestion is obviously crucial, it’s not as crucial as far as immediate survival goes as some of the other functions that the body has to carry out.
Just as in the case of psychological stressors, physiological stressors, especially starvation or semi-starvation,55 such as when eating too few calories, or partaking in exhaustive exercise,56 also contribute to leaky gut.
Leaky gut is often described as a condition in which the tight junction proteins that connect the epithelial cells making up the lining of the small intestine become destabilized, allowing bacterial toxins and large particles of undigested food from within the intestine to reach the bloodstream.
In the above image, the yellow blocks are our epithelial cells. They’re the cells that make up the lining of our gut. The spaces between them are the tight junctions. As you can see in the image, certain factors can make these gaps open up. Maintaining the stability of these tight junctions (keeping the gaps closed) is energy-dependent.57
However, there’s another reason why energy matters so much. It has been suggested that when the cell is de-energized, it loses its structural integrity, allowing particles to “leak” through it. This means that the epithelial cells themselves can become leaky, and particles can flow out of the gut and into the bloodstream through them (through the “yellow blocks” above), and not just through the tight junctions connecting them. This phenomenon is called “persorption,” and it means that any instance in which cells are de-energized will contribute to more leakiness of gut contents into the blood.
“Nasonov showed that dyes and even particles enter energetically depleted cells.” - from the article “Food-junk and some mystery ailments: Fatigue, Alzheimer's, Colitis, Immunodeficiency” by Ray Peat, PhD
“About 30 years ago some biologists made a movie of living cells under the microscope, showing an ameboid cell entering another cell, swimming around, and leaving, without encountering any perceptible resistance; persorption of food particles, moving in one side of a cell and out the other, wouldn't seem so mysterious if more people had seen films of that sort. In the 1960s, Gerhard Volkheimer rediscovered the phenomenon of persorption, which had been demonstrated a century earlier.” - from the article “Food-junk and some mystery ailments: Fatigue, Alzheimer's, Colitis, Immunodeficiency” by Ray Peat, PhD
“Persoprtion is the para-cellular passage of large corpuscular food particles through the epithelial layer of the gastro-intestinal tract.” - G. Volkheimer, “Persorption of Particles.” 1 February 196858
High estrogen levels can make the gut permeable, as can histamine (antihistamines have been shown to reduce gut permeability),59 serotonin,60 and cortisol.61 This is because they all interfere with energy creation.
“Serotonin/5-hydroxytryptamin (5-HT) produced by enterochromaffin cells in the intestine and histamine produced by mucosal mast cells act as a proinflammatory mediators in the intestine and modulate intestinal permeability”62
High serotonin levels are actually seen in most intestinal issues and serotonin-lowering drugs have been used successfully to reverse numerous digestive issues.63 Serotonin, apart from increasing gut permeability, also acts as a signalling molecule that pathogenic bacteria use to communicate with one another, multiply, become more virulent, and form biofilms that help them survive eradication attempts with antibiotics.64
“Serotonin […] can also act as a bacterial signaling molecule for pathogenic bacteria. Specifically, we found that serotonin acts as an interkingdom signaling molecule via quorum sensing and that it stimulates the production of bacterial virulence factors and increases biofilm formation.”65
Some pathogenic bacteria can also synthesize serotonin, as well as adrenaline, noradrenaline and histamine,66 which is why dysbiosis and/or an overgrowth of bacteria can contribute to nervousness, insomnia, and mental illness in general. Once again, these “stress hormones”/”stress neurotransmitters” go hand in hand, as serotonin can also cause mast cell degranulation, increasing histamine levels.
“Serotonin is believed to facilitate mast cell degranulation and migration while simultaneously being secreted by degranulating mast cells.”67
Bacterial toxins created by bad gut bacteria (such as endotoxin) can make the gut leaky too.68 These toxins tend to be created when the biome is overgrown and/or dysbiotic, and when foods that overfeed bacteria are eaten. Most people with gut issues are dealing with all three (too many bacteria, dysbiotic bacteria, and eating the wrong foods). The toxins created by these bacteria also increase histamine and serotonin levels, since, as mentioned earlier, all factors that disturb energy metabolism tend to synergize.69
Since avoiding all food can avoid feeding gut bacteria, some sources recommend fasting for leaky gut. However, the energy deficit brought on by fasting and the liberation of histamine7071 caused by going too long without food will make a leaky gut even worse once food is reintroduced. Fasting also reduces gastric motility, both during and after the fast.7273 With reduced peristalsis (as discussed earlier), bacteria can migrate up the intestine, resulting in SIBO.
“Histamine release is enhanced under extreme conditions such as dehydration or hypoglycemia or by a variety of stressors.”74
“We have investigated the influence of fasting and lactovegetarian diet on intestinal and non-intestinal permeability in 5 patients with rheumatoid arthritis. […] Both intestinal and non-intestinal permeability decreased after fasting, but increased again during a subsequent lactovegetarian diet regime.”75
The reason why gelatinous broths and collagen help with “leaky gut” is because, apart from glucose, glutamine, an amino acid found in gelatin, serves as a major fuel source for the epithelial cells that make up the gut lining,76 helping them create energy and stay in an energized state.
On the other hand, the reason why gluten can increase leaky gut in some isn’t because gluten has some malignant property that is universally bad. It has been found that gluten can increase the levels of a protein called zonulin, which can signal the opening of the tight junction proteins. However, gluten seems to do this only in individuals who have an overgrowth of certain pathogenic bacteria, including certain strains of E.coli, Shigella and Clostridium difficile. At the same time, commensal bacteria, such as Bifidobacterium bifidum, seem to prevent gluten from causing gut permeability.77787980 However, an overgrowth of any bacteria in the small intestine triggers the release of zonulin and the consequent opening up of the lining of the small intestine even in the absence of gluten, as a mechanism to flush these bacteria out of the intestine.81
“This zonulin-driven opening of the paracellular pathway may represent a defensive mechanism, which flushes out microorganisms and contributes to the host response against bacterial colonization of the small intestine.”82
This means that it’s not the gluten itself that’s the villain per se, rather, if someone has a dysbiotic microbiome that reacts to/feeds on the gluten protein, the byproducts of this bacterial activity can trigger leaky gut. This should be good news for many of the leaky gut sufferers out there as it might mean that gluten won’t have to be off the menu for you forever.
All in all, “sealing” a leaky gut is not a one-and-done deal. To prevent the leakage of gut contents that shouldn’t be in the blood into the blood, the body has to be maintained in a state of energy abundance. This is dependent on having optimal thyroid hormone production and utilization, enough micronutrients for energy creation, enough calories, and a lack of the factors that interfere with energy creation and utilization.
How Carbohydrates Can Help to Heal the Gut
Speaking of energy metabolism, thyroid function and gut health…let’s talk carbohydrates.
Nearly every single gut protocol out there blames sugar for gut issues and recommends going low carb to fix digestive problems. This is awful advice, for multiple reasons.