Is Grave's Disease the Body's Attempt to Regenerate the Thymus?
What if there is no need to suppress the thyroid indefinitely?
Disclaimer: Content for entertainment purposes only. Not medical or health advice.
I think that Grave’s disease is probably one of the scarier and more confusing diagnoses out there. In a world that is increasingly hypothyroid (this is not a figure of speech, official statistics show that our average core body temperatures are dropping), a thyroid that all of a sudden goes haywire, overproducing thyroid hormones and flooding tissues with them is a perplexing sight. Grave’s disease is the most common cause of true hyperthyroidism.
Grave’s disease can be quite frightening, as its symptoms include a very rapid heart rate, insomnia, diarrhea, heat intolerance, hyperthermia, tremors, rapid weight loss, and muscle weakness. More severe cases can lead to a condition called “thyroid storm,” which can lead to arrhythmia and in extreme cases when untreated even heart failure. Additionally, about 20% of those diagnosed with Grave’s disease develop Graves’ ophthalmopathy1, which is a protrusion of the eyes forward due to enlargement of the muscles and/or fat tissue surrounding the eyes. There are other “overgrowths” that tend to be associated with Grave’s disease, such as Graves’ dermopathy, an overgrowth and thickening of the skin, and Thyroid acropachy,2 the thickening and growth of bones.
The currently accepted view of Grave’s disease is that it is an autoimmune disease, with autoimmunity being defined as a confusion of the immune system, causing the immune system to attack and destroy parts of the body. In Grave’s disease, the immune system is perceived as attacking the thyroid gland, causing it to release an excessive amount of thyroid hormones. However, I do not think that this view is entirely correct.
What if the immune system isn’t attacking the thyroid?
Since Grave’s is believed to be caused by immune confusion that results in the immune system mistaking the thyroid gland for a bacterial/viral pathogen and developing antigens against it that it will always remember, mainstream medicine sources state that Grave’s disease is lifelong and cannot be cured but only managed.
However, there are documented cases of spontaneous remission of Grave’s disease.345
For example, a report published in the January 2024 edition of the Journal of the Endocrine Society documented the progress of 11 patients who were diagnosed with Grave’s disease at Zuyderland Medical Center in the Netherlands and refused treatment.6 In all of them, the onset of Grave’s disease was caused by a severe psychological stressor, such as a death or serious illness in the family. Psychological stress is a known trigger for the onset of Grave’s disease.78 Of the 11 patients, 9 experienced remissions without taking antithyroid drugs or receiving any treatment to directly address Grave’s disease. Their remission was brought on by stress relief alone.
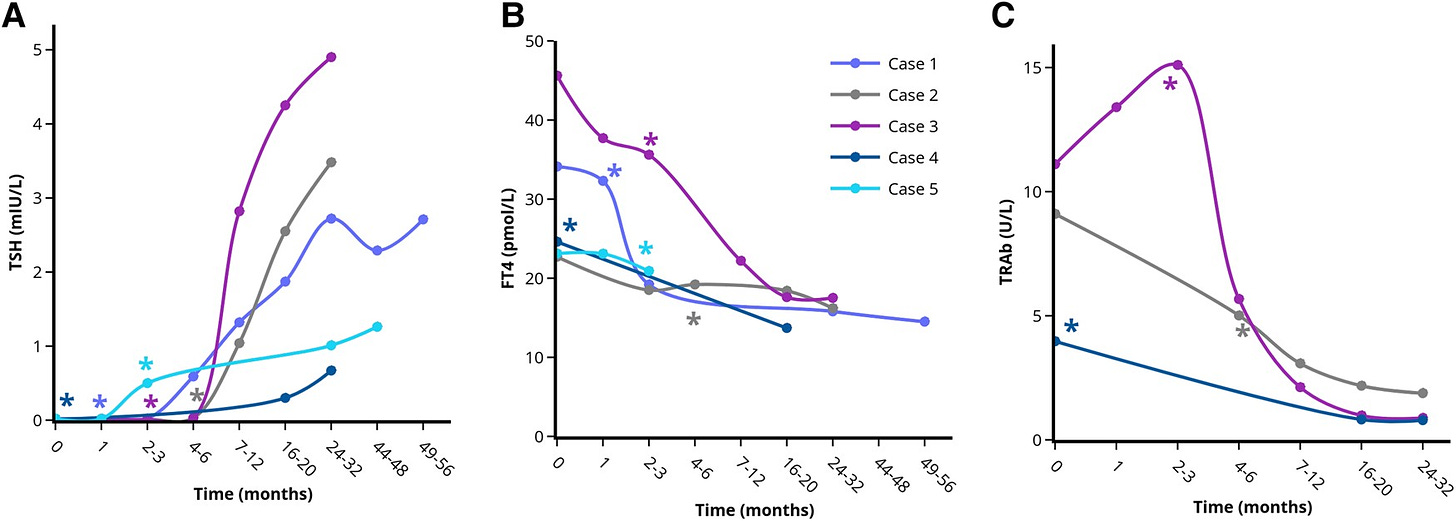
If the mechanism behind Grave’s truly was that the immune system has learned to identify the thyroid as a pathogen and will remain keen on destroying it, spontaneous remission should not be possible, yet it undeniably is.
In my article on Hashimoto’s thyroiditis, which is the first article of this series analyzing “autoimmune” thyroid disorders from the bioenergetic lens, I dive deep into the work of Dr. Jamie Cunliffe and his theory of the immune system as a system responsible for maintaining tissue homeostasis (morphostasis).9
The currently accepted view of immunity defines the immune system’s role based on its action, and its perceived action is to kill anything foreign and dangerous. Through the lens provided by this view, autoimmunity is perceived as the immune system mistaking one of the body’s organs for a foreign microbial pathogen, turning on it with the intention to kill.
Dr. Cunliffe’s model defines the role of the immune system not so much by its specific action but by its purpose. In his view, the purpose of the immune system is to maintain tissue homeostasis. This purpose can be achieved in numerous ways, including cleaning up the debris of dead cells to deny fuel to potential bacterial pathogens, stimulating the rebuilding of damaged tissues, and neutralizing pathogens.10 The perspective shift proposed in his models helps to explain many of the “paradoxes” of autoimmunity and is supported by findings such as that "autoimmune" antibodies are involved in brain repair after traumatic injury.11
The perspective shift would have to then be made away from viewing the immune system as a killing squadron intent on spotting anything foreign to the body and killing it, and towards viewing the immune system as a clean-up and repair crew.
Perhaps the reason why spontaneous remission is possible in Grave’s disease is because the antibodies found in Grave’s disease, such as the thyroid stimulating immunoglobulin (TSI) haven’t mistaken the thyroid for a pathogenic bacteria and are not attacking it.
But if they aren’t attacking the thyroid then what are they doing?
Well, based on my hours digging through PubMed, reading obscure medical literature from the 1800s, and nearly short-circuiting my brain as I tried to wrap my head around the “purpose” behind Grave’s disease (while working with the framework that the body is never trying to hurt itself and is always seeking homeostasis and repair), I have landed on the hypothesis that Grave’s disease is the body’s attempt to repair the thymus gland and return to immune homeostasis. This perspective will be explored in this article and the proof supporting it will be provided.
Shifting our perspective away from Grave’s disease being a disease of the thyroid to instead the possibility of it being a disease of the thymus would also explain the peculiarity of Grave’s goitre.
Grave’s disease, apart from its many symptoms, is also characterized by the appearance of goitre (an enlargement of the thyroid gland). Unlike the goitres that tend to form in cases of what I believe to be true disorders of the thyroid gland, where the goitre might be fibrotic and asymmetric and the output of thyroid hormones is reduced, in the case of Grave’s disease, the thyroid simply forms a goitre by becoming very large, without experiencing any fibrosis, asymmetry, or inhibition of function. If the body was attacking the thyroid, why would the thyroid appear…not injured at all… but instead just larger than normal? This question too will be explored in this article.
Why I’m writing this
I started investigating Grave’s disease after a friend of mine ended up being diagnosed with it upon experiencing some of the symptoms above. She reached out to me asking for my opinion on Grave’s disease and if I have any recommendations for what can be done.
I was already familiar with the work of Dr. Jamie Cunliffe and have seriously doubted that autoimmune conditions are caused by the body attacking itself, especially since environmental factors (such as high estrogen levels, psychological stress, high bacterial endotoxin levels produced by pathogenic gut bacteria or a recent viral or bacterial infection) are often implicated in the onset of autoimmune diseases, and all of these factors can cause excessive cellular damage and even cell death by inhibiting energy production. It would make sense then to speculate that the reason why factors that lead to tissue damage are associated with the presence of “autoimmune” antibodies is because these antibodies are involved in the cleanup and repair of injured cell components.
I have also been familiar with the perspective that perceives hyperthyroidism as a misdiagnosis of a hypothyroid state, due to anecdotes from the work of Dr. Broda Barnes who observed that patients thought to be hyperthyroid due to symptoms such as tremors, a fast heart rate and rapid weight loss were often found to have a low core body temperature upon a more detailed examination and saw improvements on desiccated thyroid therapy, revealing that their symptoms were not due to high levels of thyroid hormones but instead to high adrenaline levels.
However, the symptoms of Grave’s disease cannot be explained away by high adrenaline levels, as part of the assessment process is by measuring thyroid hormone levels, and both T4 (the thyroid prohormone) and T3 (the active thyroid hormone) are excessively elevated in Grave’s disease.
While I was convinced that the argument that explains Grave’s disease as an immune “attack” on the thyroid that causes it to produce more thyroid hormones than it normally would does not make much sense, I lacked a better explanation that could elucidate why all of a sudden the immune system was stimulating the thyroid to make more hormones.
I was able to find some information on Grave’s disease that could provide my friend with some clues, such as that high estrogen is involved in the onset of Grave’s (the ratio of those diagnosed with Grave’s disease is 5:1 women to men) and that vitamin D1213 and selenium14 have been shown to lead to remission. Yet when asked why high estrogen would lead the thyroid to overproduce hormones or why vitamin D or selenium would alleviate this the best answer that I could come up with was:
Basically, I had no clue. And it seemed that no one else had any clue either.
Since the currently-held belief is that the immune system will continue to attack the thyroid gland indefinitely, the “management” of the disease usually involves either taking a drug that suppresses the thyroid and prevents the creation of thyroid hormones for the rest of your life, or the removal of the thyroid gland and replacement therapy with levothyroxine, which is a monotherapy of the thyroid prohormone T4.
If experiencing a thyroid storm, taking a drug that prevents the excessive creation of thyroid hormones short term can be lifesaving. However, long term, it is absolutely horrendous and usually results in severe hypothyroidism, the consequences of which I have spoken of extensively (my whole Substack is basically dedicated to explaining the dangers of hypothyroidism).
The removal of the thyroid gland is an even more horrible solution, not only because it makes the person dependent on hormone replacement therapy for the rest of their life, but also because T4-only medications often make women even more hypothyroid, as estrogen can burden the liver and make it harder for women to convert T4 to the active thyroid hormone T3.
I obviously did not want my friend to have to resort to either one of these barbaric solutions, but without being able to make sense of the disease in my head, how could I help her make sense of what was going on?
I set out on a mission to make sense of the phenomenon that is Grave’s disease and answer questions such as:
Why is Grave’s disease often triggered by psychological stress or a viral/bacterial infection?
Why is estrogen implicated in the onset of Grave’s disease?
Why does the thymus tend to hypertrophy in Grave’s disease?
Why do other tissues tend to hypertrophy?
Why do vitamin D and selenium help?
I knew that whatever answer I landed upon would be a better explanation than the current one and would provide better options than just permanently nuking the thyroid. And I think that I might have solved the riddle.
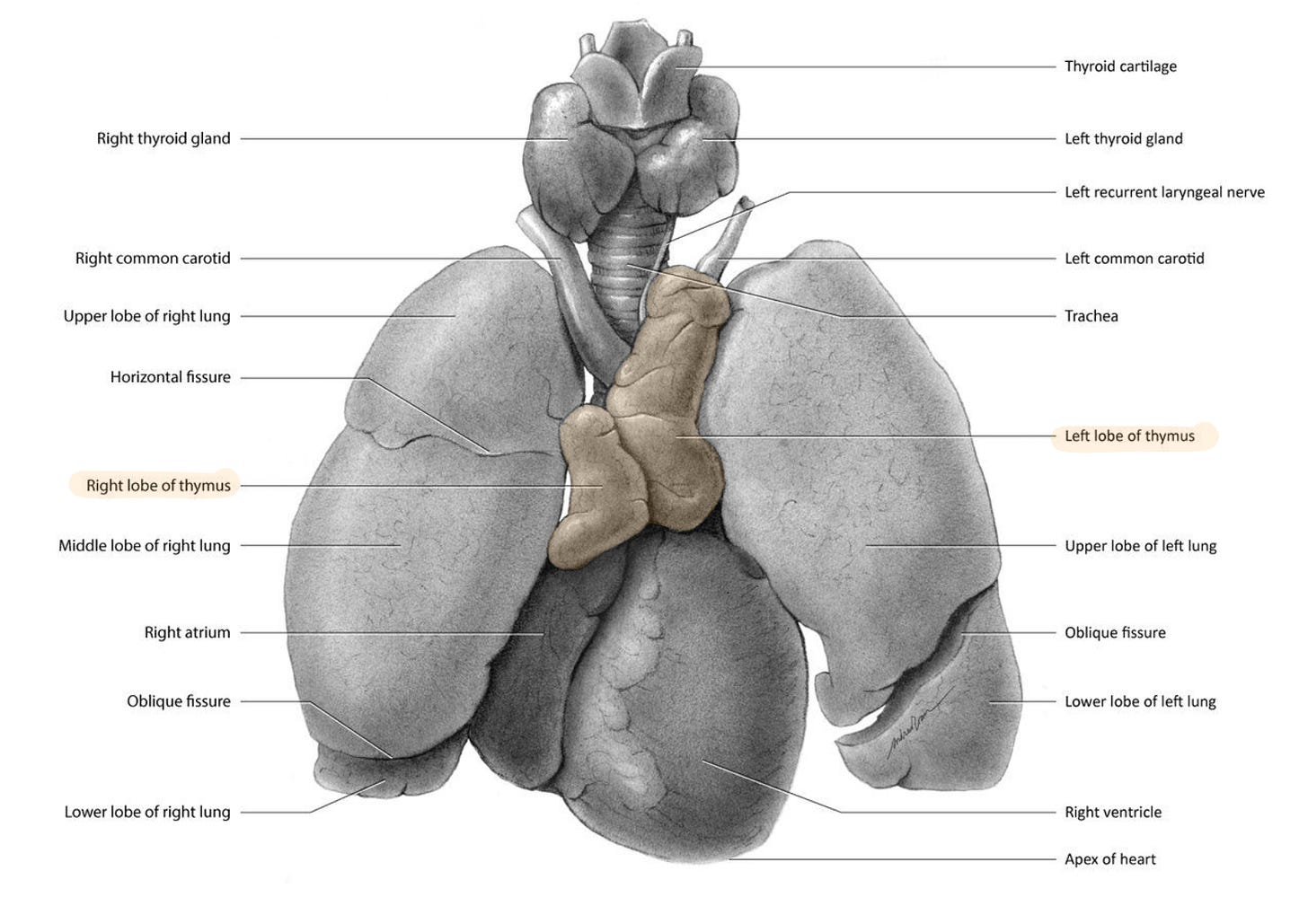
Thymus - the forgotten gland
Since so much of this article revolves around the thymus, I think it would make sense to kick things off by introducing this forgotten gland.
The thymus gland sits right behind the sternum, and it’s unique in the fact that it is part of two organ systems - the endocrine (hormonal) system and the lymphatic system.
The thymus gland plays a vital role in immunity, as it is the hub responsible for the generation and maturation of T cells. T cells are cells that are involved in the immune response, with their main goal being to spot patterns that indicate tissue danger or “mess,” memorize the pattern to better respond to it in the future, and neutralize pathogens. These cells include the T helper cells, the T regulatory cells and the “killer” T cells.
For many years, medicine held the belief that the thymus gland is no longer needed past childhood, on the observation that the thymus gland is the gland most affected by ageing, undergoing severe atrophy (shrinking) after childhood. This gave way to the belief that the thymus is redundant and non-functional in adulthood, and even that there should be no consequences to thymus removal.
The thymus gland is an extremely malleable gland, which is what contributed to this belief. Since the thymus gland can dramatically atrophy when the body’s environment gets hostile, it wasn’t uncommon to find that upon examining the bodies of older (and sick) people, their thymus glands were often nowhere to be found.
“It should always be recalled that in any wasting disease whatever, the thymus reacts very quickly and intensely and shrinks nearly to the point of disappearance. This explains why we usually, in our ordinary autopsies, find a very small thymus or fail to find it at all, while in autopsies in those instances of sudden perhaps accidental deaths the thymus is not so small and is therefore found more frequently.”15
This brought forth the belief that its disappearance in adulthood is normal. While the disappearance of the thymus gland with age may have been common, further research has illustrated that its disappearance with age is not normal and is instead a sign of sickness and a contributing factor to the aging process, making it clear that thymic depletion should not be normalized with age.
Thankfully, by now, the dogma believing that the thymus gland is not needed past childhood has been challenged. An assessment of healthy centenarians found that they still have present and functional thymus glands, disputing the idea that the thymus is meant to dissolve after puberty.16
“An immunological tenet was that the most important phenomenon of immunosenescence is the involution of the thymus. In most textbooks and papers it is taken for granted that the thymus starts its involution immediately after puberty. When people aged 60-65 were considered old, it was not difficult to think that they could live for the rest of their life with a fully involuted thymus. The findings on centenarians challenge this tenet, as they have only a small reduction of T lymphocytes, and a relatively normal number of virgin and memory T cells, together with a functional T cell repertoire.”17
Multiple studies have shown that in animals with a thymus that has atrophied or in ones missing a thymus, the growth of cancerous tumours is accelerated and the body’s ability to stop the proliferation of cancerous cells is diminished.1819 This is because mature T cells are involved in spotting and helping to dispose of cancer cells. The same was observed in humans. In a review assessing the health of patients who had their thymus removed during cardiac surgery vs those who didn’t, those whose thymuses were removed experienced a 2.9x greater risk of death, a 2x greater risk of developing cancer, and a greater risk of developing autoimmune disorders.20
In his 2006 newsletter on autoimmunity,21 Dr. Ray Peat speculated that the reason why the thymus can disintegrate and regenerate so rapidly (sometimes within hours) compared to the other organs is because it consists mainly of the cells involved in cleaning up messes and regenerating tissues (immune cells), as per the framework of Dr. Cunliffe.
When it comes to Grave’s disease, the role of the thymus cannot be overlooked as thymic abnormalities seem to go hand-in-hand with Grave’s disease.
The relationship between the thymus and Grave’s disease
The connection between the thymus and Grave’s disease is not an arbitrary one. When looking into Grave’s disease, the connection between it and the thymus gland was impossible to miss.
For one, an enlargement of the thymus gland is often seen in Grave’s disease,22232425 and the removal of the thymus has been known to bring on a remission of Grave’s disease since the late 1800s,262728 bringing additional merit to the hypothesis that Grave’s disease is not brought on by a dysfunctional thyroid but rather by a dysfunctional thymus.
“Mikulicz (1895) called attention to the occurrence of enlarged Thymus in severe cases of exophthalmic goitre;* and Rehn (1899) suggested that it might be well to attack the Thymus surgically in that disease; and Hart (1908) expressed the opinion that abnormal activity of the Thymus might of itself produce the clinical picture of Grave’s disease.”29
*”Exophthalmic” means “characterized by protruding eyes” and is a term that is often used in older literature to refer to the goitre seen in Grave’s disease.
What is interesting about the enlarged thymuses seen in Grave’s disease is that they are often abnormal in their appearance and function. For one, the thymus in Grave’s disease has been observed to contain lymphoid germinal centers,30 which are centers that produce B cells. B cells are immune cells that are implicated in “autoimmunity” (or, based on the work of Dr. Cunliffe, tissue rebuilding). Normally, the thymus does not produce B cells. The enlarged thymuses in Grave’s disease seem to also be characterized by atrophy and degeneration of Hassall’s bodies,31 which are regions in the thymus that are responsible for the maturation of T cells and the removal of cells undergoing cell death.32 This would explain the general imbalance between T cells and B cells in Grave’s disease, where B cells tend to be overproduced, whereas certain T cells, such as the thymus-derived regulatory T cells, seem to be diminished.33 This imbalance will become relevant in later parts of this article.
In some experiments, Grave’s disease was induced in an animal by transplanting into it a pathologically enlarged thymus gland.34
However, what is most interesting about the thymus and Grave’s connection is that in 1893 it was accidentally discovered that feeding patients diagnosed with Grave’s disease the raw thymus gland of a healthy young animal could bring Grave’s disease to remission.
Healing Grave’s by thymus feeding
In 1893, a British doctor by the name of David Owen prescribed to one of his patients with a history of Grave’s disease an order for a thyroid gland from the butcher. At the time, the use of thyroid glandular was the standard for treating all forms of goitre, despite being quite dangerous in the case of Grave’s disease and producing mixed results.
Since the Universe seems to operate in strange ways, the butcher accidentally ended up mixing up the order and provided the patient with a thymus gland from a healthy lamb instead. After taking a quarter of an ounce of the raw thymus gland daily for 3 months, Dr. Owen’s patient saw a disappearance of his symptoms, despite having had Grave’s disease for 20 years and failing to respond to what was at the time conventional treatment.
The happy accident propelled Dr. Owen to use the thymus preparation with four more of his patients diagnosed with Grave’s disease, each time producing successful results. The only case in which he saw no improvement was a case where the thymus gland was sourced from an old animal in wintertime, indicating that the animal’s thymus may have been defective due to disease, and improvements were seen again when a thymus gland from a young calf substituted that from the lamb.35
“The thymus was used for the first time, and accidentally at that, in 1895* by Owen, who had prescribed for his patient fresh thyroid. The doctor saw afterward only that the butcher had mistaken the thymus for the thyroid, yet the result of its use was brilliant.” - From the book: “Thyroid and Thymus” by Dr. André Crotti36
*As per Owen’s original report,37 the incident took place in 1893.
In his book “Practical Hormone Therapy: a Manual of Organotherapy for General Practitioners”38 published in 1914 by Dr. Henry R. Harrower, Dr. Harrower notes that the technique of thymus feeding to Grave’s patients developed by Dr. Owen was later successfully adopted by multiple other practitioners.
“A few years later Kinnicutt collected sixty-two cases of exophthalmic goitre treated by this method. In thirty-six cases improvement of varying degree occurred, in twenty-five there was no improvement, and in one there was an aggravation of the symptoms. After nearly twenty years S. Solis-Cohen states that he prefers thymus extract to all other organotherapeutic preparations in the treatment of exophthalmic goitre. […] ] MM. Dor of Lyons are enthusiastic about the value of thymus extracts. One of them writes that ‘nothing ameliorates exophthalmic goitre like thymus’ […] Hirsch advocates this method of treatment, and reports that thymin (tablets made from the thymus of the calf) causes an improvement in the restlessness and sleeplessness, a regression of the struma, reduction of the exophthalmos, as well as a lessening of the cardiac disturbances. She states that improvement was noticed in two cases in which operation had been without effect.” - From the book:”Practical Hormone Therapy: a Manual of Organotherapy for General Practitioners” by Dr. Henry R. Harrower
What is worth mentioning though is that with the Owen method, his patients only saw a remission of symptoms so long as they stayed consistent in their daily consumption of raw thymus, often failing to comply due to its offputting taste. This indicates that perhaps the original imbalance that was increasing these patients’ thymus requirements was not being addressed.
Why would it be that both consuming a healthy thymus but also the surgical removal of the thymus seem to bring Grave’s disease to remission in some? This will be explored later in this article, illustrating that it is not a paradoxical finding.
However, first I want to propose that Grave’s disease is triggered by any event that leads to the destruction of the thymus, and the onset of the disease is an attempt to repair the thymus that gets out of control.
Grave’s disease tends to be triggered by events that destroy the thymus gland
Viral39 or bacterial40 infections as well as psychological stress tend to be common triggers for the onset of Grave’s disease.
Simultaneously, these tend to be the same triggers that lead to the atrophy of the thymus gland. Due to the delicate nature of the thymus gland, high levels of the stress hormones adrenaline and cortisol, which tend to elevate in cases of psychological stress, are known to shrink the thymus, cause tissue fibrosis in the thymus, and deplete the pool of mature T cells. This same shrinking, depletion and fibrosis is also easily brought on by infections, low-calorie and/or low-protein diets, vitamin and mineral deficiencies and general malnutrition, partially because all of these are stressors that lead to increased levels of the adrenal hormones.4142434445
“It has been a long time since scientists noticed that, in the context of the malnutrition-related immunodeficiency, the thymus undergoes a variety of alterations, comprising, among others, a severe atrophy. This is so consistent that the thymus has been considered as a barometer of malnutrition. Interestingly, such thymic atrophy can also be found in a variety of infectious diseases.”46
The relationship between the thymus and the thyroid
There seems to be a relationship between the thymus and the thyroid where the function of each of these glands affects the other.
A deficiency of the thymus seems to accelerate the function of the thyroid, while thyroid hormones stimulate the growth of the thymus.4748 At the same time, an excess of thymic tissue seems to suppress the thyroid.49
The push-and-pull relationship between the two glands was illustrated in an experiment on tadpoles, where administering tadpoles with excess thymus tissue had such a potent anti-thyroid activity that the tadpoles never differentiated into frogs and instead just ended up increasing in size, becoming massive tadpoles. Alternatively, giving tadpoles an excess of thyroid tissue caused them to differentiate into tiny frogs without growing in size at all, showing that the thyroid’s inherent differentiation-inducing property is opposed and balanced by the thymus.50

Considering the relationship between the two glands, here is what I believe takes place in Grave’s disease:
A stressor, such as an infection, a psychological stressor, or a nutritional stressor leads to an atrophy/near disappearance of the thymus.
The resulting “thymus deficiency” functions as a signal, accelerating the function of the thyroid.
The increased output of thyroid hormones helps to regenerate the thymus.
Now, in the ideal world, once the thymus regains its shape and function, the thyroid should not have to continue to increase its output, causing the output of thyroid hormones to normalize. I believe that this is exactly what happened in those patients mentioned near the start of this article who experienced transient Grave’s disease following a stressor, with spontaneous remission once the stressor passed. The passing of a stressor likely stopped the cascade of stress hormones that would continue to dismantle the thymus.
However, what’s happening in cases where this “healing cascade” does not seem to taper off, and the thyroid continues to put out excessive thyroid hormones while the thymus keeps growing?
When the repair process goes wrong
A delicate balance needs to be maintained between the various immune cells, such as the B cells (which are produced mainly by the bone marrow), and the various types of T cells (which mature and in many cases originate in the thymus), such as the T regulatory cells, the helper T cells, and “killer” T cells. After leaving the thymus, helper T cells differentiate into different subsets of helper T cells (such as Th1, Th2 or Th17) in the spleen and lymph nodes.
B cells tend to be involved in “autoimmunity” (or tissue rebuilding, if understood through Dr. Cunliffe’s framework). A recent study from 2021 found that B cells are indeed involved in rebuilding damaged tissues,51 supporting Dr. Cunliffe’s hypothesis.
T regulatory cells are responsible for telling B cells when to stop rebuilding.
T helper cells leave the thymus without being committed to any particular “division.” Based on the hormonal and nutritional environment of the body, they get “assigned” to become either Th1, Th2, or Th17 helper T cells.
Th2 and Th17 helper T cells stimulate B cells’ rebuilding processes.
When the rebuilding process goes wrong, an imbalance in the levels of these cells is the result.
Imagine that you are building a sand castle on the beach, but the tide is so high that whenever you’re almost done building your sand castle, a big wave splashes the entirety of the beach, destroying your castle and forcing you to start again from scratch.
Sure, in the case of a sand castle, you can just come back later when the water has calmed down a bit to continue the process.
Except in this scenario, you can’t because the sand castle in question is not a sand castle at all but a vital organ in your body and you have to manage to somehow rebuild it, despite the “high tide” that seems to never go away. (Yes, the sand castle is the thymus).
Due to the “high tide” (the suboptimal environment of the body), your “sand castle” (thymus) ends up in a state of limbo, undergoing a seemingly endless loop of constant destruction and repair. What you do in that situation is try to “outrun” the water, by building faster.
This is where a dangerous feedback loop can happen.
Normally, the T regulatory cells created by the thymus keep the B cells in check, telling them that they have built enough. However, a thymus that’s stuck in a loop of destruction and repair cannot produce enough T regulatory cells to tell B cells to back off. To make matters worse, since the B cells are needed for the rebuilding of the thymus, a thymus that’s “under maintenance” produces additional B cells for its own repair (as outlined in the examples earlier that show that in Grave’s disease, the enlarged thymus produces additional B cells), furthering the imbalance between the B cells that build and the T regulatory cells that tell them “enough!”
“The thymus gland permits immune cells to mature and to become organized. It is a major factor in the regulation of the cells that produce antibodies, the B (bone marrow derived) lymphocytes, and when the thymus is chronically damaged, the production of antibodies tends to increase, but without the sensitive control the thymus provides.”- from: “Autoimmunity” by Ray Peat, PhD52
These B cells, when in excess, can constantly stimulate rebuilding - the rebuilding of the thyroid (making it grow big) and rebuilding of other tissues (which would explain the various phenomenons of “excess growth” in Grave’s disease in places where tissue damage occurred.)
Fundamentally, what happens in Grave’s disease is that there tends to be an excess of building and a deficiency of “stop building!”
“The thyroid can be functionally suppressed, and, with stimulation, return to full activity within a few hours. If it is stimulated continuously, it can increase its mass greatly in a few days.” - from: “Autoimmunity” by Ray Peat, PhD53
Of course, the prevailing view is that the TSI (thyroid stimulating immunoglobulin) created by B cells is “attacking” tissues as opposed to rebuilding them in places where damage has taken place. However, the rebuilding hypothesis provides a more convincing way to explain why in some cases of Grave’s ophthalmopathy only one eye is affected, despite both eyes having “TSH receptors” that the TSI “antibody” could bind to and “attack.”
A 2022 paper titled “The Mysterious Universe of the TSH Receptor”54 describes the curious observation that TSH receptors (which got their name due to the belief that they’re only found within the thyroid), were found in other tissues, including the pituitary, hypothalamus, kidney, adrenals, liver, the immune system, blood cells, vascular tissues, skeletal muscles, cardiac cells, bones and even fat tissue. Yet these tissues are not all systemically “attacked” by the TSI “antibodies” which are believed to attack the TSH receptor in an act of immune confusion in Grave’s disease. If Grave’s truly was an act of immune confusion where the TSI antibody targets and attacks the TSH receptor, why would it only “attack” some of these receptors in very select tissues while ignoring others?
I think these observations help to strengthen the counter-argument against the prevailing “immune confusion and attack” hypothesis and support the notion that the TSI “antibody” is instead “cleaning up” damaged tissues to restore tissue homeostasis.
Going back to the view of the immune imbalance in Grave’s being due to cellular damage, when it comes to furthering this imbalance between T regulatory cells and B cells, the hormonal and nutritional environment of the body plays a big role.
Factors such as high estrogen levels55 and bacterial endotoxin,56 as well as selenium deficiency57 (among others), can favour the “assignment” of helper T cells to the Th2 and Th17 “divisions.” As mentioned, Th2 and Th17 helper T cells increase the activity of B cells, creating a nasty feedback loop.
Additionally, the body should be able to generate additional T regulatory cells in the periphery (outside of the thymus) by converting T helper cells into T regulatory cells, but this ability is stifled in certain environmental conditions (for example, in a vitamin D5859, retinol60 or progesterone61 deficiency the body’s ability to turn helper T cells into T regulatory cells can be inhibited and instead favour the generation of Th17 cells).
Due to the environment of the body, the rebuilding cascade that started as a way to regenerate the thymus can’t stop.
This immune remodelling is not just speculative as Grave’s disease is marked by a deficiency of the anti-inflammatory T regulatory cells together with a relative increase in the number of B cells and Th2 & Th17 helper T cells, with the Th17 cells specifically being highly pro-inflammatory.62636465666768
Tying together all the loose ends
If we perceive the action of the B cells to be that of stimulating rebuilding rather than attacking self-tissue, Grave’s disease takes on the appearance of a disease brought on by a rebuilding cascade that fails to become restrained.
The idea that Grave’s is a “repair process gone south” is supported by the finding that thymic hyperplasia in Grave’s disease is more common in young patients,69 as thymic restoration is generally easier to achieve at a younger age.
This “failure in restrainment” is brought on by a thymus that is incapable of providing enough of the T regulatory cells needed to “turn off” this building cascade, in a body whose hormonal and nutritional environment stimulates excess “rebuilding.”
The fact that excess estrogen would stimulate this cascade, by contributing to Th2-type immune dominance, makes absolute sense, as estrogen is the primordial hormone involved in tissue growth. This is supported by the finding that Grave’s disease is most often diagnosed in females in their late 40s70 who are beginning to enter menopause. Menopause is a time of estrogen dominance, as ovulation ceases, preventing the creation of the corpus luteum and its resulting progesterone secretion.
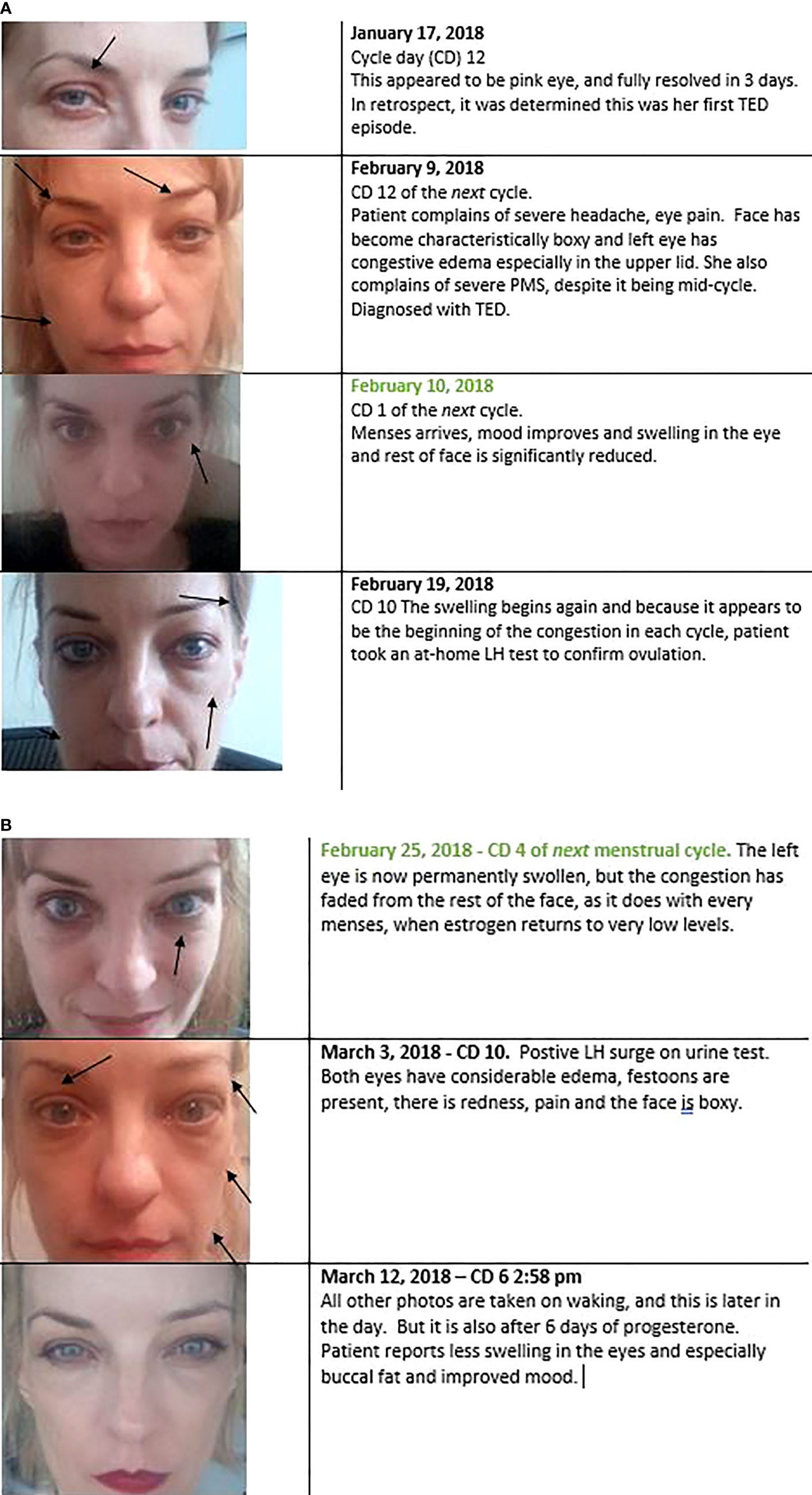
In one case study, published in a 2022 paper titled “Is Estrogen a Missing Culprit in Thyroid Eye Disease? Sex Steroid Hormone Homeostasis Is Key to Other Fibrogenic Autoimmune Diseases - Why Not This One?71,” a woman with Grave’s ophthalmopathy (Grave’s associated eye disease) experienced a protrusion of the eyes that was aggravated during ovulation, when estrogen levels tend to spike. Estrogen is a major player in tissue swelling. Her symptoms of Grave’s ophthalmopathy would improve at the beginning of her menstrual period (when estrogen levels are at their lowest) and resolved with progesterone supplementation. Photos from the case study below:
The reason why cigarette smoking is a major risk factor for developing Grave’s disease7273 is because cigarette smoking, just like estrogen, promotes Th2 immune dominance.74 Perhaps this is because the damage done to the lungs by smoking mobilizes B cells into a repair process for restoring tissue homeostasis in the lungs, or perhaps cigarettes are often contaminated with heavy metals such as cadmium, which are estrogenic in nature.
Since an abnormally hyperplastic thymus tends to produce additional B cells while its T cell production tends to be negatively affected, it makes sense why, when transplanted into animals, an abnormal thymus could bring on Grave’s disease.
It also makes sense why in certain cases of Grave’s disease removing the unhealthy thymus brought on the resolution of symptoms, likely by removing an additional source of B cells that could shift the immune system towards a “B cell dominant” state. However, since the absence of the thymus in and of itself can set off this cascade, as its removal can take away the “brake” needed to keep B cells in check, in some instances the removal of the thymus in Grave’s disease backfired, and instead of bringing on the cessation of symptoms, it caused a thyroid storm.75
As such, while removing the thymus can sometimes make the symptoms of Grave’s disease go away, it does not address the root of the issue and can often backfire, just as removing the thyroid gland can make the symptoms go away, without actually addressing the factors that are causing this immune dysregulation in the first place.
At the same time, the reason why feeding those suffering from Grave’s disease healthy thymus tissue tended to bring relief is because a healthy thymus contains T helper cells and proteins that help to convert them to T regulatory cells, which could provide extra T cells to keep the B cells restrained.76 Additionally, the thymus tissue contains various hormones and peptides, all of them with immunomodulatory actions. For example, thymosin alpha, a peptide found in thymic tissue, can increase the number of T regulatory cells.77 The thymic peptides thymulin and thymopoietin (and its synthetic analogue thymopentin) have been shown to modulate the immune response towards Th1 immunity and away from a Th2 dominant immune response.7879
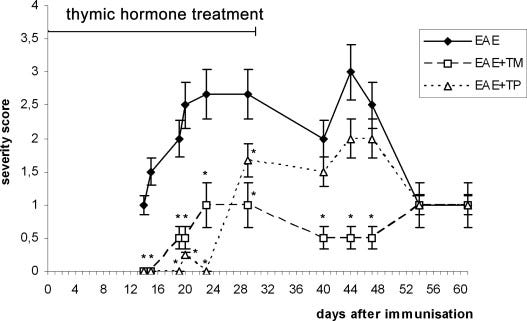
in mice with acute experimental autoimmune encephalomyelitis (EAE), which resembles multiple sclerosis in humans
In mice with experimentally induced autoimmune encephalomyelitis (EAE), a model resembling human multiple sclerosis, the administration of thymulin (noted as EAE+TM on the graph below) and thymopentin (noted as EAE+TP on the graph below), the thymic peptides reduced the severity of the disease.80
Taking all of these factors into consideration, what should help in Grave’s disease is:
Addressing estrogen dominance
Removing factors that cause fibrosis and thymic & tissue damage
Preventing Th2 immune dominance
Removing factors that favour the differentiation of T helper cells into Th17-type cells
Maximizing factors that favour the differentiation of T helper cells into inducible T regulatory cells
What can be done about this?
Removing factors that cause thymic & tissue damage
“It has been shown that radiation, polyunsaturated fatty acids, estrogens, and heavy metals and other toxins including dioxins damage the thymus gland, and can produce immunodeficiency. These stressors also stimulate the "autoimmune" antibodies.” - from: “Autoimmunity” by Ray Peat, PhD
The thymus is rapidly dismantled under stress. To repair the thymus, while preventing its repeated destruction, stay warm, calm, and well fed.
Dietary carbohydrates help to prevent the stress response, especially ones that are sweet and mineral-rich, such as ripe fruit, freshly squeezed juice, honey, and cooked pumpkins and squashes.
If dairy is tolerated, “clean” ice cream with minimal ingredients and no added gums and preservatives (such as Haagen-Dazs vanilla bean or Straus Family Creamery vanilla bean or Dutch chocolate) can be a powerful food for lowering the stress response.
Avoid going too long without food and avoid undereating, especially when recovering from an illness. For women especially, tracking their calories with an app such as Cronometer can be quite revealing, as women tend to undereat without realizing that they’re not eating enough. I would aim for at least 2,000 calories per day for any grown adult, potentially much more than that depending on hunger cues and activity level.
Nutrient deficiencies linked to thymic atrophy include zinc, copper, iron and vitamins A (retinol), B1, B2, B5, B6, B7, and E.8182
Foods such as organ meats (liver, kidney, heart), shellfish (oysters, mussels, clams), ripe fruit (oranges, mangoes, kiwis), eggs, and dairy (buffalo dairy is particularly high in biotin) provide many of these nutrients. A varied diet (for example, using various sources and types of fruit, culinary fruit, meat cuts and dairy from various animals) can increase the likelihood of meeting all these nutritional bases.
Protein deficiency is strongly linked to thymic atrophy.8384
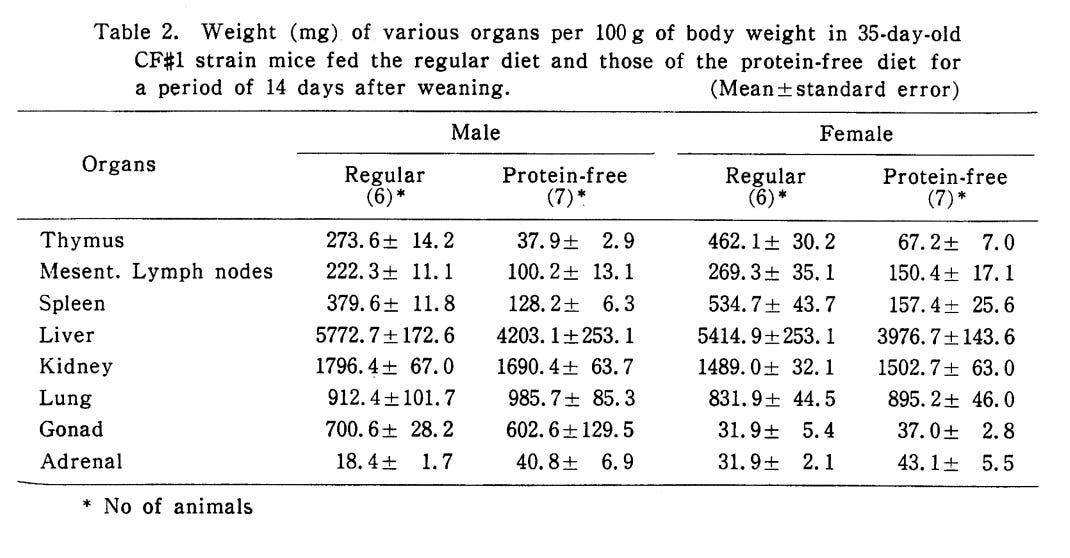
“Thymic atrophy induced by protein malnutrition is primarily due to elevated serum corticosterone.”85
Cold temperatures can cause the thymus to shrink.8687 Avoid cold baths and ice plunges. Dress warm in winter, and prioritize hot Epsom salt baths. Avoid exposing the sternum to cold temperatures, especially if sick or shortly following illness. Keeping a hot water bottle over the sternum might be helpful.
The avoidance of polyunsaturated fats, environmental estrogens, heavy metals and radiation (as outlined in Dr. Peat’s quote above) should be prioritized as well. This can be done by limiting the ingestion of fried foods, restaurant food and pre-packaged foods, limiting the use of cleaning products, makeup, cosmetics, perfumes, and fertilizers, keeping electronics away from the body, and switching to butter and coconut oil as cooking fats.
Keeping psychological stress low and keeping biological stress low (by staying well-nourished and limiting environmental toxins) should be prioritized, as high cortisol levels are the main culprit of thymic involution.
Addressing estrogen dominance
Dietary protein assists the liver with the detoxification of estrogens. Many nutrients and food compounds are involved in supporting estrogen metabolism and detoxification,8889 such as:
The amino acids taurine, glycine, methionine, cysteine and glutamine, found in animal products.90 Glycine and glutamine are predominantly found in gelatinous cuts, and taurine is high in scallops and hearts.
B vitamins (especially B2 and B6), found in foods such as liver, brewer’s yeast, eggs, lean pork, bee pollen, shellfish and ripe mangoes, cherimoyas and oranges. A B-complex supplement can be helpful. I like the one from BalancedBodyMind and the one from LifeBlud.
Magnesium, found in cacao powder, dates and oranges. Since enough magnesium can be hard to obtain via diet alone, a supplement can be helpful
Indole-3-carbinol, found in high amounts in broccoli sprouts.
Limonene, found in the peel of citrus fruits, in foods such as orange or lemon peel marmalade.
Calcium D-glucarate, found in apples and oranges.
Carnosic acid, found in rosemary
When various fibres were assessed in their ability to bind to and excrete estrogens from the colon, oat bran was half as effective as the prescription bile binder cholestyramine.91 Soaking oat bran in buttermilk or lemon water overnight can help reduce its mineral-chelating properties. Out of the vegetables tested for their bile-binding ability in a different study, steamed collard greens performed best.92 By binding to bile in the intestine (and the metabolized estrogens found in it), such a fibre can increase estrogen excretion. A daily raw carrot salad can help too.
“The in vitro binding of estrone, estradiol-17 beta, estriol, testosterone, dihydrotestosterone, and estrone-3-glucuronide by wheat, oat, and corn brans, oat hulls, cellulose, lignin, and cholestyramine resin was measured. The extent of steroid sequestration was characteristic and reproducible for each hormone. Cholestyramine bound an average of 90% of all the steroids tested, whereas cellulose bound the least (12%). Of the other substances tested, each bound the following percentage of unconjugated hormones: lignin, 87%; wheat and oat brans, 45% each; corn bran 44%; and oat hulls, 32%.”93
Since many are sensitive to wheat, oat bran can be a safer choice.
Increase the intake of nutrients and foods that inhibit the estrogen synthase (aromatase) enzyme and antagonize estrogen receptors,94959697 such as:
Vitamin E, found in extra virgin olive oil, kiwis and red peppers. Since enough vitamin E can be hard to obtain via diet alone, a supplement can be helpful.
Vitamin A, found in liver, eggs and dairy (especially goat dairy).
Vitamin K2, found in cheese (such as gouda and camembert).
Chrysin, found in propolis and honey.
Apigenin, found in chamomile, parsley and guavas.
γ-Mangostin, found in the mangosteen fruit.
Well-cooked white button mushrooms.
Coffee
Avoid foods and herbs high in phytoestrogens (such as flax seeds, soy, red clover and lavender), lower exposure to environmental xenoestrogens, and avoid polyunsaturated fats, as they can inhibit liver glucuronidation. Having daily bowel movements is paramount to estrogen clearance, and being relatively lean can help prevent estrogen dominance.
In some instances, a progesterone supplement, such as Dr. Peat’s Progest-E, can be helpful.
Preventing Th2 immune dominance
Addressing estrogen dominance will play a big factor in shifting away from a Th2-dominant immune system state. Certain food compounds that can help move away from a Th2-dominant immune response are:
Selenium, found in kidneys, lean fish, shellfish, and eggs.
Gingerol, found in ginger.98
D-Fraction beta-glucan, found in maitake mushrooms.99
Glycine, found in collagen, gelatin, and gelatinous meat cuts (oxtail, shanks, beef cheeks, chicken feet).100
Safranal, found in saffron.101
Carvacrol, found in oregano oil.
“Carvacrol treatment caused significant reduction of Th2, Th17, gene expression of IL-4, IL-17 and TGF-β but increase Treg cells, Th1/Th2 ratio, gene expressions of FOXP3 and IFN-γ and IFN-γ/IL-4 ratio (p<0.05 to p<0.001).”102
Supplementing with a desiccated thymus extract might be helpful as well, however, it should be introduced slowly to watch for how the body reacts to its immunomodulatory action. Since in Dr. Owen’s work back in the 1800s best results were seen with either lamb or veal thymus, desiccated thymus brands sourced from lamb or veal might be best. The brand Ancestral Supplements offers a desiccated sheep thymus supplement.
Lowering levels of the Th17 type T helper cells & increasing T regulatory cells
These compounds help to shift the Treg/Th17 balance in favour of T regulatory cells:
Vitamin D103
Vitamin A (retinol)104
Vitamin E105
Vitamin K2, found in cheese, liver and dark goose and chicken meat.106
Zinc, found in oysters, beef and lamb107
Progesterone
Endotoxin, a bacterial toxin produced by pathogenic gut bacteria, can favour the differentiation of T helper cells into Th17-type cells as opposed to T regulatory cells.108 A daily bowel movement is crucial in keeping endotoxin levels low.
Avoiding hard-to-digest foods that cause digestive discomfort and excessive gas can help lower endotoxin levels. These foods vary from person to person.
For those with high endotoxin levels, an avoidance of gluten-containing foods can be helpful.
Activated charcoal can help lower endotoxin levels, but it can also bind to nutrients and should be used sparingly.
Food compounds that help to protect against endotoxin-induced inflammation and the resulting Th17-type immune response include:
Myricetin, found in cranberries, blueberries and Swiss chard.109
Apigenin, found in chamomile and parsley.110
Ellagic acid and gallic acid, found in pomegranates.111
Thymulin, a thymic peptide found in thymus tissue.112
Pu-erh tea113
In conclusion…
A high metabolic rate and proper thyroid function are foundational to good health and disease avoidance. Unfortunately, Grave’s disease is often treated through the suppression of the thyroid gland or its removal. Considering that the data above suggests that Grave’s disease isn’t even a disease of the thyroid but rather of the thymus, I think that suppressing the thyroid long-term is misguided and misses the real issues at hand.
As my last concluding remark, I will throw in a screenshot from a case report114 where Grave’s disease was brought to remission with lifestyle and diet modifications alone, without the use of any anti-thyroid drugs.
Part one of this series, titled “A Different View of Hashimoto's & Autoimmunity” investigates autoimmunity and autoimmune thyroiditis in more detail and explores topics such as “Where does the belief that the body attacks itself come from?” “Does leaky gut cause Hashimoto’s?” and “Should gluten and dairy be avoided?” It also goes on a deep dive into the history of immunology and the shaky foundations on which the concept of the body attacking itself has been built. If you found this information helpful, I would encourage you to check it out.
My Substack is a reader-supported publication.
If you found this article valuable, consider becoming a paid subscriber. Paid subscribers gain access to all of my articles and podcast episodes in full, and your support allows me to continue devoting my time and effort to my research & writing.
Some of my other articles:
Bahn RS. Graves' ophthalmopathy. N Engl J Med. 2010 Feb 25;362(8):726-38. doi: 10.1056/NEJMra0905750. PMID: 20181974; PMCID: PMC3902010.
Jadidi J, Sigari M, Efendizade A, Grigorian A, Lehto SA, Kolla S. Thyroid acropachy: A rare skeletal manifestation of autoimmune thyroid disease. Radiol Case Rep. 2019 May 23;14(8):917-919. doi: 10.1016/j.radcr.2019.04.021. PMID: 31193617; PMCID: PMC6536614.
Codaccioni JL, Orgiazzi J, Blanc P, Pugeat M, Roulier R, Carayon P. Lasting remissions in patients treated for Graves' hyperthyroidism with propranolol alone: a pattern of spontaneous evolution of the disease. J Clin Endocrinol Metab. 1988 Oct;67(4):656-62. doi: 10.1210/jcem-67-4-656. PMID: 3417846.
Jeresa I A Willems, Daan J L van Twist, Robin P Peeters, Guy J M Mostard, Roderick F A Tummers-de Lind van Wijngaarden, Stress-Induced Graves Disease: Spontaneous Recovery After Stress Relief, Journal of the Endocrine Society, Volume 8, Issue 1, January 2024, bvad157, https://doi.org/10.1210/jendso/bvad157
YUKIHIRO NAGAI, TAISYU TOYA, KEN-ICHI FUKUOKA, NOBUYOSHI TANAKA, SEKIYA YANAGI, KEN-ICHI KOBAYASHI, Occurrence and Spontaneous Remission of Graves' Hyperthyroidism Preceded by Painless Thyroiditis, Endocrine Journal, 1997, Volume 44, Issue 6, Pages 881-885, Released on J-STAGE November 21, 2006, Online ISSN 1348-4540, Print ISSN 0918-8959, https://doi.org/10.1507/endocrj.44.881
Jeresa I A Willems, Daan J L van Twist, Robin P Peeters, Guy J M Mostard, Roderick F A Tummers-de Lind van Wijngaarden, Stress-Induced Graves Disease: Spontaneous Recovery After Stress Relief, Journal of the Endocrine Society, Volume 8, Issue 1, January 2024, bvad157, https://doi.org/10.1210/jendso/bvad157
Vita R, Lapa D, Trimarchi F, Benvenga S. Stress triggers the onset and the recurrences of hyperthyroidism in patients with Graves' disease. Endocrine. 2015 Feb;48(1):254-63. doi: 10.1007/s12020-014-0289-8. Epub 2014 May 23. PMID: 24853882.
Vita R, Lapa D, Vita G, Trimarchi F, Benvenga S. A patient with stress-related onset and exacerbations of Graves disease. Nat Clin Pract Endocrinol Metab. 2009 Jan;5(1):55-61. doi: 10.1038/ncpendmet1006. Epub 2008 Nov 25. PMID: 19029994.
Cunliffe J. Morphostasis and immunity. Med Hypotheses. 1995 Feb;44(2):89-96. doi: 10.1016/0306-9877(95)90076-4. Erratum in: Med Hypotheses 1995 May;44(5):428. PMID: 7596312.
Cunliffe, J. (2007), Intentional Pathogen Killing – or Denial of Substrate?. Scandinavian Journal of Immunology, 66: 604-609. https://doi.org/10.1111/j.1365-3083.2007.02017.x
Hofstetter HH, Sewell DL, Liu F, Sandor M, Forsthuber T, Lehmann PV, Fabry Z. Autoreactive T cells promote post-traumatic healing in the central nervous system. J Neuroimmunol. 2003 Jan;134(1-2):25-34. doi: 10.1016/s0165-5728(02)00358-2. PMID: 12507769.
Alhuzaim ON, Aljohani N. Effect of vitamin d3 on untreated graves' disease with vitamin d deficiency. Clin Med Insights Case Rep. 2014 Aug 13;7:83-5. doi: 10.4137/CCRep.S13157. PMID: 25187748; PMCID: PMC4133032.
Nermin A. Sheriba, Abeer A.A. Elewa, Maram M. Mahdy, Ahmed M. Bahaa El Din, Nesma A. Ibrahim, Dina A. Marawan, Tahany M. Abd El Moneim. Effect of vitamin D3 in treating hyperthyroidism in patients with Graves' disease. Egypt J Intern Med 2017;29:64–70; doi:10.4103/ejim.ejim_10_17
Lanzolla G, Marinò M, Marcocci C. Selenium in the Treatment of Graves' Hyperthyroidism and Eye Disease. Front Endocrinol (Lausanne). 2021 Jan 26;11:608428. doi: 10.3389/fendo.2020.608428. PMID: 33574798; PMCID: PMC7870989.
Hennington CW. The Thymus Question. Buffalo Med J. 1915 Jun;70(11):611-617. PMID: 36887825; PMCID: PMC8737964.
Franceschi C, Monti D, Barbieri D, Salvioli S, Grassilli E, Capri M, Troiano L, Guido M, Bonafè M, Tropea F, Salomoni P, Benatti F, Bellesia E, Macchioni S, Anderlini R, Sansoni P, Mariotti S, Wratten ML, Tetta C, Cossarizza A. Successful immunosenescence and the remodelling of immune responses with ageing. Nephrol Dial Transplant. 1996;11 Suppl 9:18-25. doi: 10.1093/ndt/11.supp9.18. PMID: 9050030.
Franceschi C, Monti D, Barbieri D, Salvioli S, Grassilli E, Capri M, Troiano L, Guido M, Bonafè M, Tropea F, Salomoni P, Benatti F, Bellesia E, Macchioni S, Anderlini R, Sansoni P, Mariotti S, Wratten ML, Tetta C, Cossarizza A. Successful immunosenescence and the remodelling of immune responses with ageing. Nephrol Dial Transplant. 1996;11 Suppl 9:18-25. doi: 10.1093/ndt/11.supp9.18. PMID: 9050030.
Shanker A. Is thymus redundant after adulthood? Immunol Lett. 2004 Feb 15;91(2-3):79-86. doi: 10.1016/j.imlet.2003.12.012. PMID: 15019273.
Shilovsky GA, Feniouk BA, Skulachev VP. Thymic Involution in Ontogenesis: Role in Aging Program. Biochemistry (Mosc). 2015 Dec;80(12):1629-31. doi: 10.1134/S0006297915120135. PMID: 26638690.
Kooshesh KA, Foy BH, Sykes DB, Gustafsson K, Scadden DT. Health Consequences of Thymus Removal in Adults. N Engl J Med. 2023 Aug 3;389(5):406-417. doi: 10.1056/NEJMoa2302892. PMID: 37530823; PMCID: PMC10557034.
Ray Peat, PhD, “Autoimmunity,” Ray Peat's Newsletter, May 2006.
Murakami M, Hosoi Y, Negishi T, Kamiya Y, Miyashita K, Yamada M, Iriuchijima T, Yokoo H, Yoshida I, Tsushima Y, Mori M. Thymic hyperplasia in patients with Graves' disease. Identification of thyrotropin receptors in human thymus. J Clin Invest. 1996 Nov 15;98(10):2228-34. doi: 10.1172/JCI119032. PMID: 8941638; PMCID: PMC507671.
Begoña Pla Peris, Pablo Abellán Galiana, Francisco Javier Maravall Royo, Agustín Ángel Merchante Alfaro, Thymic Hyperplasia and Graves Disease: A Nonincidental Association, JCEM Case Reports, Volume 1, Issue 5, September 2023, luad083, https://doi.org/10.1210/jcemcr/luad083
Nicolle MW. Pseudo-myasthenia gravis and thymic hyperplasia in Graves' disease. Can J Neurol Sci. 1999 Aug;26(3):201-3. doi: 10.1017/s0317167100000251. PMID: 10451743.
García E, García-Hierro V, Pilar Alvarez M, de la Maza L, Santos E, Pi J, Castillo L, Ruiz E. Hiperplasia tímica en una paciente con enfermedad de Graves [Thymic hyperplasia in a patient with Graves' disease]. Endocrinol Nutr. 2009 Feb;56(2):92-5. Spanish. doi: 10.1016/S1575-0922(09)70557-1. Epub 2009 May 1. PMID: 19627717.
Hennington CW. The Thymus Question. Buffalo Med J. 1915 Jun;70(11):611-617. PMID: 36887825; PMCID: PMC8737964.
DEGROOT, L. J. (1967). Remission of Myasthenia and Thyrotoxicosis After Thymectomy. Annals of Internal Medicine, 67(5), 1042. doi:10.7326/0003-4819-67-5-1042
CROTTI, A.: Thyroid and Thymus. 774 pp. Lea & Febiger, New York, 1922.
Hennington CW. The Thymus Question. Buffalo Med J. 1915 Jun;70(11):611-617. PMID: 36887825; PMCID: PMC8737964.
GUNN, A. (1964). THE THYMUS IN THYROID DISEASE. The Lancet, 284(7363), 776–778. doi:10.1016/s0140-6736(64)90558-6
Hennington CW. The Thymus Question. Buffalo Med J. 1915 Jun;70(11):611-617. PMID: 36887825; PMCID: PMC8737964.
Rezzani R, Nardo L, Favero G, Peroni M, Rodella LF. Thymus and aging: morphological, radiological, and functional overview. Age (Dordr). 2014 Feb;36(1):313-51. doi: 10.1007/s11357-013-9564-5. Epub 2013 Jul 23. PMID: 23877171; PMCID: PMC3889907.
Hansen M, Cheever A, Weber KS, O’Neill KL. Characterizing the Interplay of Lymphocytes in Graves’ Disease. International Journal of Molecular Sciences. 2023; 24(7):6835. https://doi.org/10.3390/ijms24076835
Hennington CW. The Thymus Question. Buffalo Med J. 1915 Jun;70(11):611-617. PMID: 36887825; PMCID: PMC8737964.
Thymus Feeding In Exophthalmic Goitre, David Owen, The British Medical Journal, Vol. 2, No. 1867 (Oct. 10, 1896), pp. 1017-1019 (3 pages), https://www.jstor.org/stable/20247186
CROTTI, A.: Thyroid and Thymus. 774 pp. Lea & Febiger, New York, 1922.
Thymus Feeding In Exophthalmic Goitre, David Owen, The British Medical Journal, Vol. 2, No. 1867 (Oct. 10, 1896), pp. 1017-1019 (3 pages), https://www.jstor.org/stable/20247186
“Practical hormone therapy : a manual of organotherapy for general practitioners” by Henry R. Harrower. Harrower, Henry R. (Henry Robert), 1883-. Date: 1914
Mateu-Salat M, Urgell E, Chico A. SARS-COV-2 as a trigger for autoimmune disease: report of two cases of Graves' disease after COVID-19. J Endocrinol Invest. 2020 Oct;43(10):1527-1528. doi: 10.1007/s40618-020-01366-7. Epub 2020 Jul 19. PMID: 32686042; PMCID: PMC7368923.
Bech K, Nerup J, Larsen JH. Yersinia enterocolitica infection and thyroid diseases. Acta Endocrinol (Copenh). 1977;84:87–92.
Savino W, Dardenne M, Velloso LA, Dayse Silva-Barbosa S. The thymus is a common target in malnutrition and infection. British Journal of Nutrition. 2007;98(S1):S11-S16. doi:10.1017/S0007114507832880
McMurray DN. Cell-mediated immunity in nutritional deficiency. Prog Food Nutr Sci. 1984;8(3-4):193-228. PMID: 6396715.
Bell RG, Hazell LA, Price P. Influence of dietary protein restriction on immune competence. II. Effect on lymphoid tissue. Clin Exp Immunol. 1976 Nov;26(2):314-26. PMID: 1086755; PMCID: PMC1540839.
Bodey B, Bodey B Jr, Siegel SE, Kaiser HE. The role of zinc in pre- and postnatal mammalian thymic immunohistogenesis. In Vivo. 1998 Nov-Dec;12(6):695-722. PMID: 9891234.
Elfaki Y, Robert PA, Binz C, Falk CS, Bruder D, Prinz I, Floess S, Meyer-Hermann M, Huehn J. Influenza A virus-induced thymus atrophy differentially affects dynamics of conventional and regulatory T-cell development in mice. Eur J Immunol. 2021 May;51(5):1166-1181. doi: 10.1002/eji.202048981. Epub 2021 Mar 17. PMID: 33638148.
Savino W, Dardenne M, Velloso LA, Dayse Silva-Barbosa S. The thymus is a common target in malnutrition and infection. British Journal of Nutrition. 2007;98(S1):S11-S16. doi:10.1017/S0007114507832880
Bodey B, Bodey B Jr, Siegel SE, Kaiser HE. The role of zinc in pre- and postnatal mammalian thymic immunohistogenesis. In Vivo. 1998 Nov-Dec;12(6):695-722. PMID: 9891234.
Scheiff JM, Cordier AC, Haumont S. Epithelial cell proliferation in thymic hyperplasia induced by triiodothyronine. Clin Exp Immunol. 1977 Mar;27(3):516-21. PMID: 862237; PMCID: PMC1540935.
Jaeger, K. H., Goslar, H. G., Grigoriadis, P. G., & Back, N. (1984). Anti-thyroid activity of purified thymus gland extract in male wistar rats. Pharmacological Research Communications, 16(6), 559–577. doi:10.1016/s0031-6989(84)80037-5
Gudernatsch, J. F. (1914). Feeding experiments on tadpoles. II. A further contribution to the knowledge of organs with internal secretion. American Journal of Anatomy, 15(4), 431–480. doi:10.1002/aja.1000150403
Sîrbulescu RF, Mamidi A, Chan SC, Jin G, Boukhali M, Sobell D, Ilieş I, Chung JY, Haas W, Whalen MJ, Sluder AE, Poznansky MC. B cells support the repair of injured tissues by adopting MyD88-dependent regulatory functions and phenotype. FASEB J. 2021 Dec;35(12):e22019. doi: 10.1096/fj.202101095RR. PMID: 34792819; PMCID: PMC8756564.
Ray Peat, PhD, “Autoimmunity,” Ray Peat's Newsletter, May 2006.
Ray Peat, PhD, “Autoimmunity,” Ray Peat's Newsletter, May 2006.
Vieira IH, Rodrigues D, Paiva I. The Mysterious Universe of the TSH Receptor. Front Endocrinol (Lausanne). 2022 Jul 12;13:944715. doi: 10.3389/fendo.2022.944715. PMID: 35903283; PMCID: PMC9315062.
Salem ML. Estrogen, a double-edged sword: modulation of TH1- and TH2-mediated inflammations by differential regulation of TH1/TH2 cytokine production. Curr Drug Targets Inflamm Allergy. 2004 Mar;3(1):97-104. doi: 10.2174/1568010043483944. PMID: 15032646.
Glader P, Smith ME, Malmhäll C, Balder B, Sjöstrand M, Qvarfordt I, Lindén A. Interleukin-17-producing T-helper cells and related cytokines in human airways exposed to endotoxin. Eur Respir J. 2010 Nov;36(5):1155-64. doi: 10.1183/09031936.00170609. Epub 2010 Feb 25. PMID: 20185422.
Lanzolla G, Marinò M, Marcocci C. Selenium in the Treatment of Graves' Hyperthyroidism and Eye Disease. Front Endocrinol (Lausanne). 2021 Jan 26;11:608428. doi: 10.3389/fendo.2020.608428. PMID: 33574798; PMCID: PMC7870989.
Huang, F., Ju, Yh., Wang, Hb. et al. Maternal vitamin D deficiency impairs Treg and Breg responses in offspring mice and deteriorates allergic airway inflammation. Allergy Asthma Clin Immunol 16, 89 (2020). https://doi.org/10.1186/s13223-020-00487-1
Fisher SA, Rahimzadeh M, Brierley C, Gration B, Doree C, Kimber CE, Plaza Cajide A, Lamikanra AA, Roberts DJ. The role of vitamin D in increasing circulating T regulatory cell numbers and modulating T regulatory cell phenotypes in patients with inflammatory disease or in healthy volunteers: A systematic review. PLoS One. 2019 Sep 24;14(9):e0222313. doi: 10.1371/journal.pone.0222313. PMID: 31550254; PMCID: PMC6759203.
Kim CH. Regulation of FoxP3 regulatory T cells and Th17 cells by retinoids. Clin Dev Immunol. 2008;2008:416910. doi: 10.1155/2008/416910. PMID: 18389070; PMCID: PMC2278288.
Lee JH, Ulrich B, Cho J, Park J, Kim CH. Progesterone promotes differentiation of human cord blood fetal T cells into T regulatory cells but suppresses their differentiation into Th17 cells. J Immunol. 2011 Aug 15;187(4):1778-87. doi: 10.4049/jimmunol.1003919. Epub 2011 Jul 18. PMID: 21768398; PMCID: PMC3155957.
Hansen M, Cheever A, Weber KS, O’Neill KL. Characterizing the Interplay of Lymphocytes in Graves’ Disease. International Journal of Molecular Sciences. 2023; 24(7):6835. https://doi.org/10.3390/ijms24076835
Yi Wang, Sijie Fang, Huifang Zhou, Pathogenic role of Th17 cells in autoimmune thyroid disease and their underlying mechanisms, Best Practice & Research Clinical Endocrinology & Metabolism, Volume 37, Issue 2, 2023, 101743, ISSN 1521-690X, https://doi.org/10.1016/j.beem.2023.101743.
Liu HY, Shi ZY, Fan D, Zhang SX, Wu LX, Lu KY, Yang SY, Li WT, Kang JF, Li CH, Cheng ZH, Xue Y, Wu ZF, Li XF, Li SJ. Absolute reduction in peripheral regulatory T cells in patients with Graves' disease and post-treatment recovery. Mol Immunol. 2022 Apr;144:49-57. doi: 10.1016/j.molimm.2022.02.004. Epub 2022 Feb 18. PMID: 35189399.
Kocjan T, Wraber B, Repnik U, Hojker S. Changes in Th1/Th2 cytokine balance in Graves' disease. Pflugers Arch. 2000;440(5 Suppl):R94-5. PMID: 11005626.
Phenekos C, Vryonidou A, Gritzapis AD, Baxevanis CN, Goula M, Papamichail M. Th1 and Th2 serum cytokine profiles characterize patients with Hashimoto's thyroiditis (Th1) and Graves' disease (Th2). Neuroimmunomodulation. 2004;11(4):209-13. doi: 10.1159/000078438. PMID: 15249726.
Vannucchi G, Covelli D, Currò N, Dazzi D, Maffini A, Campi I, Bonara P, Guastella C, Pignataro L, Ratiglia R, Beck-Peccoz P, Salvi M. Serum BAFF concentrations in patients with Graves' disease and orbitopathy before and after immunosuppressive therapy. J Clin Endocrinol Metab. 2012 May;97(5):E755-9. doi: 10.1210/jc.2011-2614. Epub 2012 Mar 7. PMID: 22399512.
Kustrimovic N, Gallo D, Piantanida E, Bartalena L, Lai A, Zerbinati N, Tanda ML, Mortara L. Regulatory T Cells in the Pathogenesis of Graves’ Disease. International Journal of Molecular Sciences. 2023; 24(22):16432. https://doi.org/10.3390/ijms242216432
Hennington CW. The Thymus Question. Buffalo Med J. 1915 Jun;70(11):611-617. PMID: 36887825; PMCID: PMC8737964.
Barajas Galindo DE, Ramos Bachiller B, González Roza L, García Ruiz de Morales JM, Sánchez Lasheras F, González Arnáiz E, Ariadel Cobo D, Ballesteros Pomar MD, Rodríguez IC. Increased incidence of Graves' disease during the SARS-CoV2 pandemic. Clin Endocrinol (Oxf). 2023 May;98(5):730-737. doi: 10.1111/cen.14860. Epub 2022 Dec 30. PMID: 36510647; PMCID: PMC9877771.
FitzPatrick AM. Is Estrogen a Missing Culprit in Thyroid Eye Disease? Sex Steroid Hormone Homeostasis Is Key to Other Fibrogenic Autoimmune Diseases - Why Not This One? Front Immunol. 2022 Jun 17;13:898138. doi: 10.3389/fimmu.2022.898138. PMID: 35784325; PMCID: PMC9248759.
Prummel MF, Wiersinga WM. Smoking and risk of Graves' disease. JAMA. 1993 Jan 27;269(4):479-82. PMID: 8419666.
Wiersinga WM. Smoking and thyroid. Clin Endocrinol (Oxf). 2013 Aug;79(2):145-51. doi: 10.1111/cen.12222. Epub 2013 May 11. PMID: 23581474.
de Heens GL, van der Velden U, Loos BG. Cigarette smoking enhances T cell activation and a Th2 immune response; an aspect of the pathophysiology in periodontal disease. Cytokine. 2009 Sep;47(3):157-61. doi: 10.1016/j.cyto.2009.05.006. Epub 2009 Jul 17. PMID: 19616447.
Shoja MM, Nunez Lopez O, Okereke I. Acute Thyroid Storm Following Thymectomy: A Surprising Result of Undiagnosed Graves' Disease. Cureus. 2018 Aug 31;10(8):e3239. doi: 10.7759/cureus.3239. PMID: 30410845; PMCID: PMC6209514.
Boeru V, Manea E, Olariu M. The in vitro effect of a thymic polypeptidic extract on the function of T-cells and macrophages. Endocrinologie. 1987 Apr-Jun;25(2):83-9. PMID: 2957784.
Yang X, Qian F, He HY, Liu KJ, Lan YZ, Ni B, Tian Y, Fu XL, Zhang J, Shen ZG, Li J, Yin Y, Li JT, Wu YZ. Effect of thymosin alpha-1 on subpopulations of Th1, Th2, Th17, and regulatory T cells (Tregs) in vitro. Braz J Med Biol Res. 2012 Jan;45(1):25-32. doi: 10.1590/s0100-879x2011007500159. Epub 2011 Nov 30. PMID: 22245858; PMCID: PMC3854146.
Braga M, Gianotti L, Gentilini O, Fortis C, Consogno G, Di Carlo V. Thymopentin modulates Th1 and Th2 cytokine response and host survival in experimental injury. J Surg Res. 1996 May;62(2):197-200. doi: 10.1006/jsre.1996.0195. PMID: 8632639.
S.M. Lunin, O.V. Glushkova, M.O. Khrenov, T.V. Novoselova, S.B. Parfenyuk, E.E. Fesenko, E.G. Novoselova, “Thymic peptides restrain the inflammatory response in mice with experimental autoimmune encephalomyelitis,” Immunobiology, Volume 218, Issue 3, 2013, Pages 402-407, ISSN 0171-2985, https://doi.org/10.1016/j.imbio.2012.05.023.(https://www.sciencedirect.com/science/article/pii/S017129851200126X)
S.M. Lunin, O.V. Glushkova, M.O. Khrenov, T.V. Novoselova, S.B. Parfenyuk, E.E. Fesenko, E.G. Novoselova, “Thymic peptides restrain the inflammatory response in mice with experimental autoimmune encephalomyelitis,” Immunobiology, Volume 218, Issue 3, 2013, Pages 402-407, ISSN 0171-2985, https://doi.org/10.1016/j.imbio.2012.05.023.(https://www.sciencedirect.com/science/article/pii/S017129851200126X)
McMurray DN. Cell-mediated immunity in nutritional deficiency. Progress in Food & Nutrition Science. 1984 ;8(3-4):193-228. PMID: 6396715.
Stoerk HC, Zucker TF. Nutritional Effects on the Development and Atrophy of the Thymus. Proceedings of the Society for Experimental Biology and Medicine. 1944;56(2):151-153. doi:10.3181/00379727-56-14629
“Effect of Protein Deficiency on Lymphoid Organs in Mice.” By. SEIJI KATO, SUSUMU TOMONAGA, KOTARO IHARA and KAZUHIKO AWAYA. Department of Anatomy, Yamaguchi ty School of Medicine, Ube 755, Japan, March 20, 1978, Okajimas Folia Anat. Jpn., 55(4) : 229-240, Oct. 1978
McMurray DN. Cell-mediated immunity in nutritional deficiency. Progress in Food & Nutrition Science. 1984 ;8(3-4):193-228. PMID: 6396715.
Barone KS, O'Brien PC, Stevenson JR. Characterization and mechanisms of thymic atrophy in protein-malnourished mice: role of corticosterone. Cell Immunol. 1993 Apr 15;148(1):226-33. doi: 10.1006/cimm.1993.1105. PMID: 8495490.
Savino W, de Moura-Campos LC, Santa-Rosa GL. Cold as an agent to induce thymic involution in the golden hamster. Anat Anz. 1982;152(3):239-43. PMID: 7158803.
Moura-Campos LC, Savino W. Morphometrical analysis of the thymus from mice submitted to low temperature. Acta Anat (Basel). 1988;132(1):9-11. doi: 10.1159/000146544. PMID: 3400425.
HALL, DOUGLAS. (2001). Nutritional Influences on Estrogen Metabolism. Appl Nutr Sci Reports. 1.
Zhu BT, Loder DP, Cai MX, Ho CT, Huang MT, Conney AH. Dietary administration of an extract from rosemary leaves enhances the liver microsomal metabolism of endogenous estrogens and decreases their uterotropic action in CD-1 mice. Carcinogenesis. 1998 Oct;19(10):1821-7. doi: 10.1093/carcin/19.10.1821. PMID: 9806165.
Hodges RE, Minich DM. Modulation of Metabolic Detoxification Pathways Using Foods and Food-Derived Components: A Scientific Review with Clinical Application. J Nutr Metab. 2015;2015:760689. doi: 10.1155/2015/760689. Epub 2015 Jun 16. PMID: 26167297; PMCID: PMC4488002.
Shultz TD, Howie BJ. In vitro binding of steroid hormones by natural and purified fibers. Nutr Cancer. 1986;8(2):141-7. doi: 10.1080/01635588609513887. PMID: 3010251.
Kahlon TS, Chiu MC, Chapman MH. Steam cooking significantly improves in vitro bile acid binding of collard greens, kale, mustard greens, broccoli, green bell pepper, and cabbage. Nutr Res. 2008 Jun;28(6):351-7. doi: 10.1016/j.nutres.2008.03.007. PMID: 19083431.
Shultz TD, Howie BJ. In vitro binding of steroid hormones by natural and purified fibers. Nutr Cancer. 1986;8(2):141-7. doi: 10.1080/01635588609513887. PMID: 3010251.
Renuka Mani, Vijayakumar Natesan, Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action, Phytochemistry, Volume 145, 2018, Pages 187-196, ISSN 0031-9422, https://doi.org/10.1016/j.phytochem.2017.09.016.
Chen S, Oh SR, Phung S, Hur G, Ye JJ, Kwok SL, Shrode GE, Belury M, Adams LS, Williams D. Anti-aromatase activity of phytochemicals in white button mushrooms (Agaricus bisporus). Cancer Res. 2006 Dec 15;66(24):12026-34. doi: 10.1158/0008-5472.CAN-06-2206. PMID: 17178902.
Balunas MJ, Su B, Brueggemeier RW, Kinghorn AD. Natural products as aromatase inhibitors. Anticancer Agents Med Chem. 2008 Aug;8(6):646-82. PMID: 18690828; PMCID: PMC3074486.
Kotsopoulos, J., Eliassen, A.H., Missmer, S.A., Hankinson, S.E. and Tworoger, S.S. (2009), Relationship between caffeine intake and plasma sex hormone concentrations in premenopausal and postmenopausal women. Cancer, 115: 2765-2774. https://doi.org/10.1002/cncr.24328
Ahui ML, Champy P, Ramadan A, Pham Van L, Araujo L, Brou André K, Diem S, Damotte D, Kati-Coulibaly S, Offoumou MA, Dy M, Thieblemont N, Herbelin A. Ginger prevents Th2-mediated immune responses in a mouse model of airway inflammation. Int Immunopharmacol. 2008 Dec 10;8(12):1626-32. doi: 10.1016/j.intimp.2008.07.009. Epub 2008 Aug 8. PMID: 18692598.
Inoue A, Kodama N, Nanba H. Effect of maitake (Grifola frondosa) D-fraction on the control of the T lymph node Th-1/Th-2 proportion. Biol Pharm Bull. 2002 Apr;25(4):536-40. doi: 10.1248/bpb.25.536. PMID: 11995941.
Aguayo-Cerón KA, Sánchez-Muñoz F, Gutierrez-Rojas RA, Acevedo-Villavicencio LN, Flores-Zarate AV, Huang F, Giacoman-Martinez A, Villafaña S, Romero-Nava R. Glycine: The Smallest Anti-Inflammatory Micronutrient. International Journal of Molecular Sciences. 2023; 24(14):11236. https://doi.org/10.3390/ijms241411236
Feyzi R, Boskabady MH, Seyedhosseini Tamijani SM, Rafatpanah H, Rezaei SA. The Effect of Safranal on Th1/Th2 Cytokine Balance. Iran J Immunol. 2016 Dec;13(4):263-273. PMID: 27999238.
“T helper cells subtypes and their cytokine gene expression affected by carvacrol in sensitized mice administered during sensitization period,” Mohammad Hossein Boskabady, Majid Kianmehr, Vahideh Ghorani, European Respiratory Journal Sep 2017, 50 (suppl 61) PA4922; DOI: 10.1183/1393003.congress-2017.PA4922
Ji J, Zhai H, Zhou H, Song S, Mor G, Liao A. The role and mechanism of vitamin D-mediated regulation of Treg/Th17 balance in recurrent pregnancy loss. Am J Reprod Immunol. 2019 Jun;81(6):e13112. doi: 10.1111/aji.13112. Epub 2019 Apr 18. PMID: 30903715.
Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007 Jul 13;317(5835):256-60. doi: 10.1126/science.1145697. Epub 2007 Jun 14. PMID: 17569825.
Huey KA, Fiscus G, Richwine AF, Johnson RW, Meador BM. In vivo vitamin E administration attenuates interleukin-6 and interleukin-1beta responses to an acute inflammatory insult in mouse skeletal and cardiac muscle. Exp Physiol. 2008 Dec;93(12):1263-72. doi: 10.1113/expphysiol.2008.043190. Epub 2008 Jun 27. PMID: 18586856; PMCID: PMC2814304.
Zhang M, Miura T, Suzuki S, Chiyotanda M, Tanaka S, Sugiyama K, Kawashima H, Hirano T. Vitamin K2 Suppresses Proliferation and Inflammatory Cytokine Production in Mitogen-Activated Lymphocytes of Atopic Dermatitis Patients through the Inhibition of Mitogen-Activated Protein Kinases. Biol Pharm Bull. 2021;44(1):7-17. doi: 10.1248/bpb.b20-00079. PMID: 33390552.
Schmitt AK, Puppa MA, Wessels I, Rink L. Vitamin D3 and zinc synergistically induce regulatory T cells and suppress interferon-γ production in mixed lymphocyte culture. J Nutr Biochem. 2022 Apr;102:108942. doi: 10.1016/j.jnutbio.2022.108942. Epub 2022 Jan 19. PMID: 35063658.
Vasilescu C, Berger D, Buttenschön K, Seidelmann M, Beger HG. Endotoxin-induced release of interleukin 6 and interleukin 1 beta in human blood is independent of tumor necrosis factor alpha. Eur Surg Res. 1996;28(1):55-62. doi: 10.1159/000129440. PMID: 8682145.
Zhang N, Feng H, Liao HH, Chen S, Yang Z, Deng W, Tang QZ. Myricetin attenuated LPS induced cardiac injury in vivo and in vitro. Phytother Res. 2018 Mar;32(3):459-470. doi: 10.1002/ptr.5989. Epub 2017 Dec 7. PMID: 29214686.
Berköz M, Ünal S, Karayakar F, Yunusoğlu O, Özkan-Yılmaz F, Özlüer-Hunt A, Aslan A. Prophylactic effect of myricetin and apigenin against lipopolysaccharide-induced acute liver injury. Mol Biol Rep. 2021 Sep;48(9):6363-6373. doi: 10.1007/s11033-021-06637-x. Epub 2021 Aug 16. PMID: 34401985.
BenSaad LA, Kim KH, Quah CC, Kim WR, Shahimi M. Anti-inflammatory potential of ellagic acid, gallic acid and punicalagin A&B isolated from Punica granatum. BMC Complement Altern Med. 2017 Jan 14;17(1):47. doi: 10.1186/s12906-017-1555-0. PMID: 28088220; PMCID: PMC5237561.
Lunin SM, Khrenov MO, Novoselova TV, Parfenyuk SB, Novoselova EG. Thymulin, a thymic peptide, prevents the overproduction of pro-inflammatory cytokines and heat shock protein Hsp70 in inflammation-bearing mice. Immunol Invest. 2008;37(8):858-70. doi: 10.1080/08820130802447629. PMID: 18991101.
Su-Jin Kim. “The Anti-inflammatory Mechanism of Pu-erh Tea via Suppression the Activation of NF-κB/HIF-1α in LPS-stimulated RAW264.7 Cells,” Biomed Sci Letters 2023;29:58-65 Published online June 30, 2023; https://doi.org/10.15616/BSL.2023.29.2.58
Brogan K, Marcelino G, Pedro C, Siefert A. Healing of Graves' Disease Thorough Lifestyle Changes: A Case Report. Adv Mind Body Med. 2019 Spring;33(2):4-11. PMID: 31476135.









Thank you for your thorough research. It is a delight to read. Please update us on your friend's health and let us know if she is improving!
What brands cacao powder do you recommend that test low/no heavy metals and contaminents? In the United States :)