How the Carnivore Diet Can Worsen Your Oxalate Problem
And What You Can Do Instead to Solve Oxalate Issues
My Substack is a reader-supported publication. To gain access to all of my articles and podcast episodes in full and to allow me to continue devoting my time and effort to my writing, consider becoming a paid subscriber ($15 per month or $99 for a yearly subscription).
The full version of this article is for paid subscribers only.
Content for entertainment purposes only. Not medical or health advice.
Oxalic acid (oxalate) is a plant defence chemical and a byproduct of metabolism that, when not detoxified, can lead to mitochondrial dysfunction, kidney stones, rashes, kidney damage, and joint and bone pain. Calcium oxalate kidney stones (which form when oxalic acid binds with calcium) are the most common type of kidney stone.1
I think oxalates are probably one of the most misunderstood and misrepresented topics in nutrition.
You either have those who seem completely unaware of the presence of dietary oxalate (sometimes in massive amounts) in the foods they recommend or those who fear all plant foods, even those with minimal or no oxalate content.
Vegan diets promote green juice cleanses, often featuring heaps of blended raw spinach, a very high-oxalate food.
Paleo diets encourage large amounts of sweet potatoes, spinach, and almond-based products, all of which are oxalate-rich.
Standard food-pyramid-based nutritional advice encourages the consumption of lots of raw vegetables, legumes and whole grains, overlooking their oxalate content.
Conversely, the carnivore or meat-based diet community tends to swing to the absolute other extreme of the spectrum, overstating the concern with dietary oxalates and insisting that all plant foods are to be feared and avoided like the plague, even those with benign levels of oxalates.
I agree that it’s a bad idea to make extremely high oxalate foods (like spinach or almond flour) a daily staple, as the oxalate content of these foods is very high and will become a nutritional burden that the body will have to deal with.
However, almost nobody acknowledges that an oxalate problem is mostly a metabolic problem. Some publications estimate that up to 90% of the body's oxalate burden comes from oxalate that is endogenously created by the liver when the body is in a suboptimal metabolic state, as opposed to originating from food.2
“Urinary oxalate is mostly of endogenous origin, and only ~10% derive from the daily nutritional intake.”3
While many voices in the carnivore community create food fear around foods with a relatively benign oxalate content, such as carrots or mashed potatoes, they fail to acknowledge that the body can create oxalates out of meat proteins when certain important vitamins and minerals are deficient and that breakdown products of glucose, fats, vitamin C and proteins can all serve as building blocks for endogenous oxalate creation, particularly when energy metabolism is impaired. Ketogenic diets (including the carnivore diet) cause impairment in energy metabolism, especially when done long-term.
If someone’s oxalate problem is due to metabolic impairments that cause their body to overproduce oxalate from proteins and breakdown products of fats and glucose (which we make in the body even if we eat zero carbs), as opposed to being caused by food sourced oxalates, going keto can exacerbate the problem.
Unlike the carnivore diet rhetoric, my goal isn’t to make someone scared of a long list of foods. Quite the opposite, as various food-derived compounds grant us protection against both dietary oxalate and the oxalates that the body can create.
The goal here is to accurately reflect the issue and show that it can (and should) be addressed in ways that aren’t extreme and that don’t demonize an entire food group or macronutrient.
The first part of this article will explain what oxalates are, how they cause damage, and how certain metabolic and nutritional factors favour oxalate generation.
The second part will go over practical tips for lowering the body’s oxalate load and improving kidney health, without going to dietary extremes.
Why I’m Writing This
Just a quick word for anyone who might be new here. First, since the internet nutrition space seems to be dominated by carnivores arguing with vegans, I want to point out that I am neither.
Second, I do want to acknowledge that if someone’s ability to handle dietary oxalate is reduced due to nutritional deficiencies, metabolic impairments, or an environmental toxic load, I absolutely see how a carnivore diet that cuts out all plant foods and thus all dietary oxalate could bring short-term relief, especially for someone coming from a background of eating an extremely oxalate rich and nutrient deficient diet. I do empathize a lot with you if you just so happen to be in this situation.
However, I also cannot ignore all the literature showing that ketogenic diets tend to favour kidney stone formation and impair oxalate excretion.
“The ketogenic diet (KD) is a low-carbohydrate and high-fat diet […] The most frequently occurring undesired outcomes of this diet are nutrient deficiencies, the formation of kidney stones, loss of bone mineral density, increased LDL (low-density lipoprotein) cholesterol levels and hormonal disturbances.”4
With a clean conscience, I would not be able to suggest such a diet to anyone dealing with an oxalate issue. Unfortunately, the recommendation to follow a carnivore diet seems to be the only recommendation that the alternative health world dishes out to those dealing with oxalate issues (or with suspected “oxalate issues," as the symptoms of oxalate excess do overlap with other disease states).
Apart from the fact that making people scared of any food that’s not ribeye contributes to the development of obvious orthorexia, restricting a diet to nothing but meat can and does create deficiencies and metabolic impairments that worsen an oxalate issue or cause it to develop in the first place. I have seen way too many anecdotes of people first developing symptoms of oxalate intoxication after following a carnivore diet. Unfortunately, these anecdotes often get explained away as the body finally “detoxing” oxalate after months of a “poison-free” carnivore diet. However, from what we know about the body’s biochemistry and what contributes to oxalate generation, it can’t be ruled out that the diet itself is furthering the problem.
I want to provide an alternative solution, one that gets to the crux of the issue without creating 10 new problems along the way.
In this article:
What Are Oxalates?
How Oxalates Cause Damage
How to Know If You Have an Oxalate Problem
How a Carnivore Diet Can Worsen an Oxalate Problem
Carnivore Diets Make Us Worse at Turning Glucose Into Energy
How Being Bad Carb Burners Makes Us Produce More Oxalates
Keto Diets Harm the Kidneys
The Consequences of Excessive Fat Burning
Acidic Diets Favour Kidney Stone Formation
Iron Overload Can Harm the Kidneys
The Consequences of Excess Protein
Keto & Dehydration
Should We Be Worried About Oxalates in Food?
Notable Food Sources of Oxalate
Vitamin C & Oxalates
How Nutrient Deficiencies Contribute to an Oxalate Problem
How the Microbiome Contributes to an Oxalate Problem
How Fungal Problems Increase Oxalate Levels
Fat Malabsorption Leads to Excess Dietary Oxalate Absorption
What Causes the Body to Overproduce Oxalate?
How Metabolic Issues Contribute to an Oxalate Problem
Undereating & Kidney Health
How Polyunsaturated Fats Can Worsen an Oxalate Problem
How Food Additives and Contamination Can Contribute to Oxalate Problems
A Better Way to Protect Ourselves Against Oxalates
7 Steps to Protect Ourselves Against Oxalates
What Are Oxalates?
Oxalic acid is a compound that is found in plants but can also be formed in the body from vitamin C, proteins, and breakdown products of polyunsaturated fats and improper glucose metabolism. The terms “oxalic acid” and “oxalate” are generally used interchangeably.
Plants use oxalates to regulate minerals, detoxify toxic metals and as protection against herbivores.5 In humans, oxalate is considered a byproduct of metabolism and “unavoidable garbage,” of sorts, as it is currently not known to have any beneficial roles in humans. That being said, some animal experiments indicate that some amount of oxalate might be involved in helping the kidneys take up sodium, chloride and water.6
While oxalate can also bind to toxic metals (like aluminum and lead) in humans, it hasn’t been shown to help us eliminate these metals, and in many cases can make them less soluble and reinforce their storage in tissues.7 On the other hand, oxalate’s ability to bind to minerals like calcium, iron and magnesium, rendering them unusable and contributing to mineral deficiencies, makes it an anti-nutrient.8
How Oxalates Cause Damage
To preface this, I want to say that, while the body has no use for oxalates and while they are harmful, under favourable dietary and metabolic circumstances, we don’t produce much of them, and those coming in from food are effectively detoxified. However, when excess oxalate is produced/consumed, and when it’s not adequately detoxified, oxalic acid and calcium oxalate (the compound formed when oxalic acid binds with calcium) become harmful in numerous ways.
Since oxalic acid is a form of acid, it can also be irritating to the cells in the mouth and intestinal tract by being mildly corrosive.
Calcium is oxalic acid’s favourite mineral and one that oxalate binds to most readily. The degree of damage caused by oxalate depends on where in the body it ends up binding calcium. Binding calcium in the gut makes oxalate less dangerous. Binding calcium outside the gut makes it more dangerous.
If oxalic acid binds with dietary calcium or magnesium in the gut, it forms a complex with them which can then be passed out of the body through a bowel movement. While this prevents oxalate from entering the general circulation where it can cause more damage, it can contribute to mineral deficiencies, as the calcium, magnesium, or another mineral that binds to oxalate in the gut will be excreted in a bowel movement instead of being absorbed.
However, if oxalic acid enters the general circulation, either from absorption in the gut or from being produced in the body, and binds to calcium in the blood, it becomes significantly more harmful.
There’s always calcium in our blood, as the blood is the delivery system that the body uses to send minerals to places where they need to do their jobs. By binding up calcium in the blood, oxalate can disrupt calcium-dependent functions in the body, like blood clotting, the maintenance of healthy blood vessel lining, and nerve function, by preventing calcium from getting delivered to its “job posts.”9
Being bad at metabolizing carbs makes us create more oxalates, and oxalates in turn further interfere with carb metabolism and the body’s ability to make energy by acting as a mitochondrial toxin with an inhibitory effect on mitochondrial complexes involved in energy creation.101112
Oxalates can also increase the levels of reactive oxygen species.13 By increasing oxidative stress, high levels of oxalate can contribute to atherosclerosis, hypertension, and general inflammation.14
When oxalic acid binds with calcium in the blood or tissues, the two create insoluble calcium oxalate crystals. While oxalic acid mostly causes biochemical damage, the sharp calcium oxalate crystals can cause physical damage when deposited in tissues. When deposited in the joints, they can cause joint pain and stiffness.15 When deposited in the kidneys, they can injure kidney blood vessels and settle in the kidneys as calcium oxalate kidney stones.16 In humans, oxalate crystals have even been found in thyroid gland tissue, where they can contribute both to hypothyroidism (including Hashimoto’s) and hyperthyroidism, by damaging the gland.17
The kidneys are the main target of calcium oxalate-induced damage, and they’re where most of the crystals get deposited.
“Metabolic alterations can lead to oxalate accumulation in body tissues, which is toxic. The kidney is a primary target of oxalate toxicity and its main excretory organ.”18
Since calcium oxalate crystals can damage tissues by literally cutting them up, they also contribute to local mast cell degranulation in the tissues that they’re damaging.19 This is because mast cells respond to all types of damage in the body.
How to Know If You Have an Oxalate Problem
You can request a blood or urine test for oxalic acid at a doctor’s office, or to get more comprehensive results, do an organic acids test (OAT) where you can see the levels of oxalic acid and oxalate precursors, like glyceric acid and glycolic acid. An OAT test can also give some clues as to whether you might have certain nutritional deficiencies or fungal infections that could be leading to increased oxalate production in the body.
However, you can also monitor certain symptoms.
Since the kidneys tend to be the main target of oxalate deposition, kidney-associated symptoms can be somewhat telling of a high oxalate burden.
The presence of kidney stones can be a big tell-tale sign, as calcium oxalate kidney stones are the most common type of kidney stone.20 Approximately 80% of kidney stones are calcium oxalate stones mixed with calcium phosphate stones. The other types of kidney stones, uric acid, struvite and cystine stones, account for approximately 9%, 10% and 1% of stones, respectively.21
While not a dead giveaway that oxalates are involved, impaired kidney function can be a sign of high oxalate levels. Some signs of impaired kidney function are high creatinine on a blood test, reduced eGFR on a blood test, swelling around and below the eyes, swollen ankles, hands and feet, hard bumps under the skin (calcium deposits), pain during urination, persistent “back pain” under the rib area (this can actually be kidney pain), nausea, lack of appetite, blood in urine, or foamy or cloudy urine. Issues with peeing, like excessive peeing, or a constant urge to pee with very little pee coming out, can be other signs. The buildup of toxins in the body due to reduced kidney function can also lead to trouble sleeping, issues with concentrating, and very dry and itchy skin.
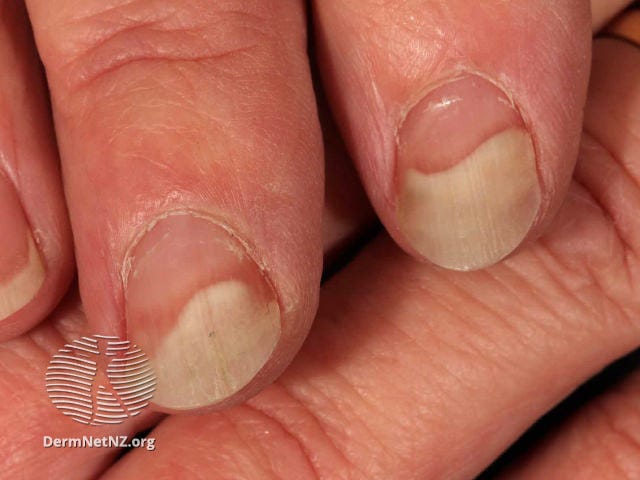
Some people with kidney disease will also develop nails that are half white, half pink,22 like this:
Others might develop nails that detach at the tip,23 creating a white fingernail tip, like this (although fungal infections can also cause this):
Another sign of excess calcium oxalate stone formation, one straight out of a horror film, can be the appearance of skin rashes and sharp crystals exiting the skin. These tend to differ quite a lot in appearance from person to person. Some manifest as skin fibrosis, others as small, raised cysts, and others look almost like open wounds, sometimes with brown crystals peeking through.2425
Since oxalate crystals can settle in various tissues, tingling, nerve pain, joint pain, connective tissue pain, and stiffness can be other signs of high oxalate levels.
The above are are some of the symptoms that are more specific and can be more telling of an oxalate issue, especially if many of these are present together. However, since oxalates can disrupt mitochondrial function (interfering with the body’s ability to make energy) and increase oxidative stress, high oxalate levels can lead to various symptoms of metabolic dysfunction and general inflammation, like fatigue, brain fog, hypertension, fat gain and even diabetes type 2.26
Symptoms of calcium depletion and electrolyte imbalances, like arrhythmia, bone and tooth pain, muscle cramps, brittle nails, tremors, depression and weak bones/teeth can also be linked to high oxalate levels.
Unfortunately, all of those symptoms are quite broad, and conditions of impaired energy production (like diabetes or hypothyroidism) have numerous root causes. Oxalates aren’t always a contributing factor.
Even calcium deficiency symptoms might not necessarily be induced by high oxalate levels. They can also be caused by toxic metals, like lead, displacing calcium, low calcium intake, or low vitamin D intake. Even excess fat in the blood, either from the overconsumption of fat, obesity, or from high adrenaline levels liberating excess fat into the blood, can cause a systemic calcium deficiency, as free fatty acids can bind to calcium and make it unavailable.27 Defects of the parathyroid gland can be another reason for low blood calcium levels, even in the absence of high oxalate levels.
Some people can also have genetic mutations that impair their ability to detoxify oxalates, which can be revealed via gene testing. The genes affected are the AGXT, HOGA1 and GRHPR genes. For example, in people with mutations in the AGXT gene, high oxalate levels come from excess conversion of protein to oxalate in the liver. High intakes of vitamin B6 can sometimes make up for this gene defect.28 In cases of high oxalate levels caused by gene mutations, symptoms first appear at 2-3 years of age, so it should be rare for someone to carry these mutations their whole life without noticing a problem until adulthood.29
How a Carnivore Diet Can Worsen an Oxalate Problem
At first glance, a carnivore diet might seem like the most logical approach to avoiding oxalates. Oxalates are found in plant foods and none are found in meat (with the exception of some species of edible snails, which are high in oxalate).30
However, the issue is not quite so simple as the body’s biochemistry is more complex.
Carnivore, ketogenic, or generally high-fat diets can paradoxically increase the body’s oxalate burden, with this effect having a lot to do with these types of diets being generally terrible for the metabolism. A slow, “backed up” metabolism is what primes the body to create more oxalates while reducing its ability to get rid of them.
Carnivore Diets Make Us Worse at Turning Glucose Into Energy
How is this relevant? It’s relevant because struggling to efficiently turn glucose into energy increases the production of oxalate precursors.
At the crux of every chronic, metabolic disease is being bad at burning glucose for energy. This is seen to a greater or lesser degree in cancer, diabetes, chronic fatigue, autoimmune diseases, and so on. People often turn to keto/carnivore diets because they are bad at burning glucose for energy, as indicated by symptoms like chronically high blood glucose levels or fat gain when eating carbohydrates.
If your body is bad at burning glucose and you severely restrict glucose (by going on a keto diet), you might see short-term improvements in symptoms, but these results are rarely long-lasting. Sadly, you’re not solving the core issue. Instead, you’re making it worse.
Ketogenic diets put the body in a chronic state of insulin resistance, forcing cells to excessively rely on burning fats for energy and making the body even worse at handling glucose,3132 an effect that can persist to some degree even after discontinuing the high-fat diet.33
Even if we eat zero carbohydrates, our livers always produce glucose (to maintain our blood glucose and to produce a steady energy supply for the brain and nervous system), so following a diet that progressively worsens our ability to burn the very glucose that our bodies will always be exposed to is counterproductive.
How Keto Diets Make Us Worse Carb Burners
“KD [ketogenic diets] lead to glucose intolerance and insulin resistance. […] Despite an initial weight loss, KD did not result in weight loss after 22 wk [weeks]. Plasma markers associated with dyslipidemia and inflammation (cholesterol, triglycerides, leptin, monocyte chemotactic protein-1, IL-1β, and IL-6) were increased, and KD-fed mice showed signs of hepatic steatosis [fatty liver disease] after 22 wk of diet. Long-term KD resulted in glucose intolerance that was associated with insufficient insulin secretion from β-cells [pancreatic cells]. After 22 wk, insulin-stimulated glucose uptake was reduced. […] Our data show that long-term KD causes dyslipidemia, a proinflammatory state, signs of hepatic steatosis [fatty liver], glucose intolerance, and a reduction in β- and α-cell mass [reduction in pancreatic cells], but no weight loss.”
- doi: 10.1152/ajpendo.00453.2013
Since glucose is our cells’ preferred fuel, forcing cells to excessively rely on fat burning has drawbacks as it is not fully compatible with our cellular machinery.
High-fat diets have been shown to turn down the expression of genes that stimulate cellular respiration (the process through which cells convert glucose into usable energy), reducing the function and assembly of mitochondrial complexes.343536 Our ability to extract energy from the foods that we eat is dependent on the activity of our mitochondrial complexes, and high-fat diets (over 40% of daily calories coming from fat), have been shown to turn down their function and assembly by up to 60%.37 In simpler terms, high-fat diets modulate our genes in a way that makes our cells worse at turning glucose (and fuel in general) into energy.
Our pancreas is where the insulin that the body uses to manage blood sugar levels is produced. Ketogenic diets have been shown to shrink the pancreas, lower the number of insulin-releasing cells, and make these cells worse at releasing insulin.38
Ketogenic-type diets also lower the level of the active thyroid hormone.39 Since thyroid hormones control the speed at which our cells turn food into energy, low levels of this hormone slow down our ability to burn glucose.
Low-carb diets worsening glucose metabolism is not a controversial concept. It is well-known in keto and carnivore circles that if you wish to undergo the oral glucose tolerance test (OGTT) at a doctor’s office (where a doctor asks you to drink a preparation containing lots of glucose to see how much your blood glucose elevates afterwards, as a way to test how well your tissues take up and burn glucose for energy), you need to increase your carb intake to at least 150 grams of carbohydrates daily for at least a few days before the test. Otherwise, if you go from eating 0 grams of carbs to undergoing the glucose tolerance test, your blood glucose will spike massively and stay elevated for a few hours due to reduced glucose-handling capacity. The doctor will then flag you as diabetic.40
In practical terms, to get the 150 grams of dietary carbohydrates needed for a normal glucose response, you’d have to eat over half a kilogram of cooked rice daily.
“With the emergence of glycated hemoglobin as a diagnostic test for diabetes, oral glucose tolerance tests (OGTTs) have become rare in endocrinology practice. As they have moved out of favor, the importance of patient instructions on preparation prior to OGTT has faded from memory. Decades-old literature, well-known to endocrinologists a generation ago, emphasized the importance of carbohydrate intake prior to OGTT. […] An OGTT performed in a research setting without adequate carbohydrate intake at the evening meal prior to the OGTT [resulted in] elevated plasma glucose levels at 1-hour and 2-hours mimicked the loss of first-phase insulin release seen in early type 1 and type 2 diabetes. […] Repeat OGTT was normal after adequate carbohydrate intake (>150 grams/day and >50 grams the evening prior to overnight fast for the study).”41
How Being Bad Carb Burners Makes Us Produce More Oxalates
Ideally, when glucose enters cellular respiration (the process through which cells convert it into usable energy), this process should proceed quickly and completely. If this process slows, stalls or becomes blocked at some step, for example, due to factors like excessive reliance on fat burning, deficiencies of key nutrients like B1 or magnesium, low levels of thyroid hormones, low intracellular oxygen levels, or the presence of toxins or heavy metals interfering with cells’ functions, byproducts of “disfigured” glucose breakdown, such as glycolate and glyoxal accumulate.42 You may already recognize glyoxal from my article on glycation, as glyoxal is also a glycating agent.
LDH-A, Oxidative Stress, and Oxalate Production
Under oxidative stress, glycolate and glyoxal can further convert to a compound called glyoxylate, which can then convert to oxalate.
The enzyme that converts glyoxylate to oxalate is called lactate dehydrogenase a (LDH-A). Being bad at burning glucose ups the function of LDH-A.43 Here is why:
If glucose enters the first stage of cellular respiration but can’t get further because the process is slowed or blocked, the cell will cut its losses and ferment it to lactate instead, to be able to get at least a little energy out of this glucose molecule (2 units of energy vs. the ideal 36-38, which it could have extracted if it managed to burn it fully). Unfortunately, the enzyme that converts glucose to lactate, when glucose burning is slow, is the same enzyme that turns glyoxylate to oxalate: LDH-A.44 In simpler terms, in cellular conditions where glucose burning is hampered, the conversion of oxalate precursors to oxalate will be increased. Among other factors, the down-regulation of mitochondrial complexes seen on high-fat diets impairs cells’ ability to rapidly move glucose through the cell.
A person who is bad at burning glucose will also experience more oxidative stress. This is in part because when the body becomes slow at burning glucose, glucose gets stuck in the blood instead of entering cellular respiration, resulting in high blood glucose levels. The body always tries to keep blood levels of all molecules (including glucose) very balanced and tries to avoid a scenario where blood glucose remains higher than optimal for long periods. When the body can’t get glucose into cellular respiration, it will try to lower blood glucose in other ways. It might try to dispose of excess glucose by sending it down a back-up cellular pathway, such as the polyol pathway. The drawback is that as a side effect of this pathway, more glyoxal can be produced.
The disposal of excess glucose through the polyol pathway (when the body struggles to burn it) is stressful and uses up antioxidants. This can lead to a depletion of antioxidants and an accumulation of reactive oxygen species. Reactive oxygen species turn glycolate and glyoxal to glyoxylate, and they can injure polyunsaturated fats (PUFAs), turning them into more glyoxal.
On low carbohydrate diets, more of the polyunsaturated fats stored in body fat get released into the blood, providing more substrate for oxalate creation.
“Another potential precursor of oxalate is glyoxal, an alpha-oxoaldehyde which can be generated from the glycation of proteins or from lipid peroxidation from hyperglycemia in diabetes.”45
Apart from turning PUFAs into glyoxal and increasing the conversion of glycolate and glyoxal to glyoxylate, reactive oxygen species also up the function of LDH-A, increasing the conversion of glyoxylate to oxalate.46
Type 2 diabetics (people who are bad at burning glucose for energy) are known to have higher levels of glyoxal and glyoxylate and to produce more oxalate.47 We don’t want to experience very high blood sugar levels chronically, especially since the kidneys are used to get rid of this excess glucose to restore blood homeostasis, and this can damage the kidneys. However, chronically high blood glucose is a symptom of being bad at burning glucose, and it’s not something that’s meant to happen when we eat carbs.
When we are good at burning glucose for energy, we create fewer reactive oxygen species, and less glycolate, glyoxal and glyoxylate. Being bad at burning glucose makes us create more reactive oxygen species, more oxalate precursors and more oxalates.
Carnivore or ketogenic diets, long term, make us worse glucose burners which, again, is a problem because our liver will always create glucose and send it into our blood for distribution, to keep us alive.
Messing with our ability to burn glucose isn’t the only way in which a carnivore diet (or a ketogenic diet more generally) can aggravate an oxalate problem. Ketogenic diets can impair our ability to get rid of oxalates by worsening kidney health.
Keto Diets Harm the Kidneys
In one experiment, where mice were placed on a high-fat diet (over 60% of daily calories as fat), after sixteen weeks on the diet, they experienced kidney injury as a result of oxidative stress and mitochondrial dysfunction (aka as a result of energy shortage).48 These effects are not unique to rodents. One case report titled “Is Losing Weight Worth Losing Your Kidney: Keto Diet Resulting in Renal Failure,” documents the case of a 36-year-old woman with no previous history of kidney problems who developed kidney damage after following a ketogenic diet for weight loss.49
Ketogenic diets or high-fat diets have been documented to harm the kidneys, through a few different mechanisms, including hampering energy generation, increasing oxidative stress, and increasing the acid load on the kidneys. Increased kidney stone formation is a known risk of ketogenic diets.50
Excessively relying on fats as an energy source can end up depriving cells of cellular energy. Fats generate overall a smaller cellular energy yield, while also causing more cellular injury in the process (I’ll expand on this in this section). Every cell in the body needs a steady stream of cellular energy to be able to maintain its function, structure and repair. This also applies to kidney cells.
When kidney cells lack energy, they become more prone to damage and lack the resources to maintain their function, reducing their ability to filter damaging particles, like oxalates, out of the body.
Let’s dive into why a ketogenic or carnivore diet can harm the kidneys. Some of these ways are more direct (such as increasing acid load), while others are more systemic (such as hampering cellular energy generation by increasing oxidative stress), but all will be covered.
Excessive Fat Burning Increases Oxidative Stress
“Oxidative stress” refers to the damage to cell structures that can take place when too many reactive oxygen species (ROS) accumulate.51 Reactive oxygen species are potentially harmful byproducts of energy creation that the body has to get rid of with antioxidants to prevent them from causing damage to the cell. Oxidative stress is known to damage the kidneys, reducing their ability to filter out oxalates.52
Excessive reliance on burning fats for energy generates more reactive oxygen species than burning glucose.5354 As mentioned earlier, forcing cells to burn predominantly fats has consequences as it is not fully compatible with our cellular machinery.
High-fat diets have also been shown to lower the expressions of genes that govern the body’s ability to make antioxidants.55
In the graphs below, you can see the results of what happened when rats were fed a standard vs. a ketogenic diet. The ketogenic diet increased levels of malondialdehyde (MDA) in their kidneys and livers (which is a compound created from injured PUFAs, and a marker and perpetrator of oxidative stress), and massively decreased superoxide dismutase (SOD) levels, which is an antioxidant that the body makes.56
When we overproduce reactive oxygen species, especially when our antioxidant defence system is impaired, they can damage cells, impair energy production, and contribute to oxalate creation.
Part of the reason why burning fats for energy creates more reactive oxygen species is that reactive oxygen species tend to form when fuel-burning happens too slowly. Fats are a slow-burning fuel, which is why our muscles use fat as fuel when at rest and not when actively engaging in exercise, a time during which they switch to glucose, which is a fuel that can be rapidly burned.
Our cells have many functions that require rapid energy generation, but trying to use fats as a rapid energy source can eventually deplete our cells of oxygen, stalling their ability to make energy and increasing reactive oxygen species generation.
It Takes More Oxygen to Burn Fats
Why are fats a slow-burning fuel? Part of the reason is that the process of burning fats for energy inside the cell tends to be more complicated than that of burning glucose.57
However, an even bigger reason is that fats are more “expensive” to burn, as burning them for energy requires more oxygen than burning glucose.
Cells need oxygen to make energy, and they need to make CO2 to get oxygen into the cell. CO2 is the “currency” that cells use to “buy” oxygen (Bohr/Haldane Effect). Apart from the fact that fat burning consumes more oxygen, burning a molecule of fat also generates less CO2 than burning glucose, leaving cells with less of the “currency” needed to buy oxygen.58
“Less CO2 is generated for every oxygen consumed when using fat over carbohydrates as fuel.”59
Chronic reliance on high-fat diets is known to induce hypoxia (cellular oxygen deficiency), as cells’ need for oxygen exceeds cells’ ability to get enough oxygen into the cell.6061 The inability to get oxygen into the cell quickly enough slows the speed at which cells can make energy.
Hypoxia itself is a big inducer of LDH-A, the enzyme that increases oxalate production.62
Since fat is a slow-burning fuel, and since our cells need to rapidly generate fuel for many of their functions, trying to rapidly burn this slow-burning fuel can eventually deplete the oxygen that cells need to make energy. As a result, cellular respiration slows and stalls further and more reactive oxygen species are generated. The resulting stressed cellular environment favours the creation of oxalate from breakdown products of glucose, proteins, and fats.
Burning Mostly Fats for Energy Creates an Energy (ATP) Shortage
While it may seem that fats give us more energy than glucose (as some fatty acids contain more ATP, the energy molecule, than glucose), the slowness of fat burning means that at the end of the day, we don’t get to make as much energy as we would from glucose.
For example, this paper63 calls out that one molecule of glucose can provide us with a maximum of 38 units of ATP (cellular energy). To extract this energy from a glucose molecule, you need 12 oxygen atoms. In the end, the ratio of energy per oxygen atom ends up being 3.1. On the other hand, one molecule of palmitic acid (a type of fat) can provide us with a maximum of 129 units of ATP. At first glance, 129 units of energy seems like a much better deal than 38. However, to get these 129 units of ATP, 46 atoms of oxygen are needed, resulting in a ratio of energy per oxygen atom of 2.8, lower than that when glucose is burned for energy.
As an analogy, imagine that you are given two job offers to choose from: Job A and Job B. At Job A, each task you complete earns you $50. At Job B, each task you complete earns you $15.
The $50 per task at Job A might seem like the better deal until you realize that the task at Job A takes one hour to complete, while each task at Job B takes 15 minutes to complete. Even though you’d make less money per task at Job B, you can complete the tasks faster which, in the end, earns you $60 an hour ($10 more than Job A).
Similarly, even though one molecule of fat might provide more ATP in theory, in practice, glucose oxidation is more rapid, costs us less oxygen, and makes more CO2, generating more ATP overall.64 Fat burns slower and is “more expensive” to burn, costing us more oxygen, and creating less of a “profit” in return (less CO2, less energy per molecule of oxygen).
As we “spend” more than we “earn” (needing more oxygen than we can afford since our share of CO2 produced is decreased), eventually cells might not be able to “afford” enough oxygen if we excessively rely on fat burning. This shows up as failing to get oxygen into the cell, resulting in hypoxia, which…upregulates LDH-A and the synthesis of oxalate.
In short, over-reliance on fat as a fuel source can eventually cause our “expenses” to be higher than our “profits.” In the body, this shows up failing to get enough oxygen into the cell, not being able to create adequate energy rapidly enough to meet our needs, and exceeding our cells’ capacity to repair themselves and maintain their function.
Carnivore Diets Are Acidic and Favour Kidney Stone Formation
“There have been several reports about renal calculi [kidney stones] developing in children on the ketogenic diet since the first report more than 30 years ago. The prevalence of renal caculi in people on the ketogenic diet is 3-10%, compared with 1 in several thousand in the general population. Chronic acidosis, dehydration, low urine pH, and fat malabsorption all contribute to the formation of uric acid and calcium oxalate stones.”65
Cutting out all plant foods and eating nothing but meat contributes to mineral imbalances and changes in the body’s pH that can damage the kidneys and favour the generation and retention of oxalate.
Yes, I know, some might have a knee-jerk reaction reading this headline because the phrase “meat is acidic” is mostly viewed as a string of nonsense that gets repeated into oblivion by vegans as if it were gospel.
However, the vegans aren’t entirely wrong here. The mineral content of different foods dictates whether they are more acidic or alkaline, and this dictates how they will affect the kidneys. The mineral composition of meat just so happens to make it acidic.
The potential renal acid load (PRAL)66 is a metric used to measure how many acid vs. base-producing nutrients a food contains, and how much of a net acid or base (alkaline) load it puts on the kidneys. Diets that are more acidic have a higher overall PRAL score.
Acid precursors include chlorine, phosphorous, sulphate, and protein (specifically the amino acids cysteine, methionine, taurine, lysine and arginine, all found in meat and animal products). Base (alkaline) precursors include calcium, magnesium, bicarbonate and potassium.67
Foods that have more of the former nutrients are more acidic. This includes foods like meat, eggs and most grains, legumes and nuts. Foods that have more of the latter nutrients are more basic (alkaline). This includes goat milk, fruit and most vegetables.
If someone eats nothing but meat (or meat and eggs), they will be getting almost exclusively only acid-forming nutrients (phosphorus, sulphate, proteins), and very little of the alkaline ones (calcium, potassium, magnesium).
The body maintains a very tight balance on its pH and the detractors of this vegan statement are correct in pointing out that blood pH rarely falls outside of the optimum range, no matter what we eat. However, this is because the body has backup mechanisms to maintain this perfect pH at all costs no matter what.
The body likes an equilibrium and will always do what it takes to keep the blood at an optimal pH, between 7.35 and 7.45. An optimal blood pH is needed for the function of the body’s enzymes and metabolic processes, so maintaining it is of utmost importance to the body.
However, what does the body use to maintain this pH? It uses nutrients (proteins and minerals), and nutrients have to come from somewhere! Since different foods contain different mineral and protein ratios, vegans aren’t wrong when they claim that what we eat can make the body more or less “acidic.” If the foods we eat mostly provide acidic minerals and proteins, the body will have to get more alkaline (base) minerals from somewhere to maintain optimal pH. Where does it get these minerals from? Mostly from dissolving our bones.
Unfortunately, the bone dissolution and extra tricks that the body has to perform to keep its pH balanced when the nutrients it gets from food are mostly acidic aren’t without consequences.
Having to pull minerals from bones to maintain pH is incredibly stressful for the body. Diets with a high acid load contribute to loss of bone mass and a greater risk of fractures and osteoporosis. The process of pulling calcium from bones is dependent on keeping parathyroid hormone (PTH) high. Parathyroid hormone is a signal of stress, and so it tends to increase the other hormones of the stress cascade, like cortisol. This has further consequences. Among other things, high cortisol levels contribute to the loss of muscle mass, insulin resistance, and fatty liver.

Apart from the negative effects on bones and systemic metabolic health, it’s the kidneys that mostly reap the consequences of acidic diets. A high dietary acid load is known to worsen kidney function over time and increase the risk of developing chronic kidney disease.686970717273
Why Acidic Diets Harm the Kidneys and Favour Kidney Stone Formation
Unlike blood pH, which is pretty stable, the pH of urine can be influenced a lot more by our diet. A diet high in mostly acidic nutrients will produce more acidic urine.
To balance out the acidic proteins and minerals when alkaline nutrients are deficient in the diet, the body will start leeching calcium from bones, considerably increasing the amount of calcium that makes it into the blood and has to be filtered out by the kidneys.
Oxalate crystals (a type of kidney stone) form when calcium binds with oxalate, which is a lot more likely to happen when excess calcium makes it into the blood and then to the kidneys, creating more opportunities for it to bind with oxalate.
Oxalate crystals can dissolve, and when that happens, we can just pee the oxalates out, but the crystals won’t dissolve when urine is too acidic.
The main compound that the body uses to inhibit oxalate crystal formation is citrate.74 Citrate can normally bind to calcium in urine, stopping it from binding with oxalate. However, when eating keto, citrate becomes deficient because:
Dietary citrate comes mostly from citrus fruits, which aren’t eaten on keto.
In the body, citrate is produced in the mitochondria, during cellular respiration (energy creation) at the step of the Krebs cycle. Keto diets lower Krebs cycle activity, decreasing citrate synthesis.7576
Normally, quite a bit of citrate remains in the kidneys and makes it into the urine, where it helps prevent the formation of oxalate crystals. However, during times of acidosis, this citrate gets reabsorbed instead. When this happens, there is little to no citrate left in the kidneys and urine to stop calcium and oxalates from combining. This gives them free rein to form crystals and stones in the kidneys.
The reason why citrate gets reabsorbed in times of acidosis is that it can be converted into bicarbonate, which is alkaline and can be used to offset all the acidic nutrients and help keep blood pH more optimal.77 Again, the body has backup pathways to maintain optimal blood pH, but these pathways come with tradeoffs and consequences. This time, the consequence is kidney stones.
Acidosis, by placing extra stress on the body as it’s forced to use multiple backup mechanisms to sort out its pH, also induces oxidative stress and hampers energy generation, further contributing to kidney injury.78